Figures & data
Figure 1. The Kaplan–Meier plot of drug survival in patients with moderate-to-severe psoriasis from the DERMBIO registry treated with biologics (Citation2).
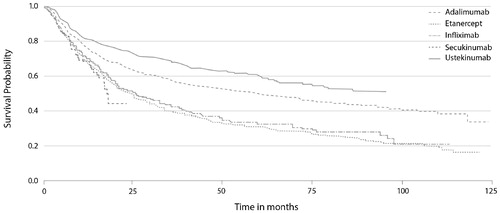
Figure 2. Comparison of TNF neutralization activity of SB4 and etanercept reference product (40 lots of EU-sourced product and 40 lots of US-sourced product), with the dotted line indicating the similarity range based on results of etanercept reference product obtained from the EU (Citation35).
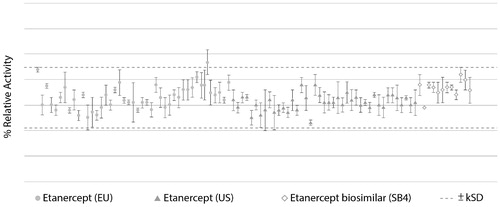
Figure 3. Psoriasis Area and Severity Index (PASI) scores during 6 months of treatment with etanercept biosimilar SB4 in patients from the PsoBiosimilars registry switched from reference etanercept and in etanercept-naive patients (Citation45). *p<.05 vs. baseline.
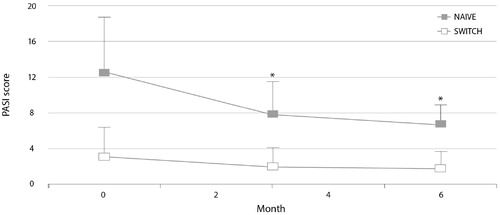
Figure 4. The Kaplan–Meier plot of drug survival in patients with moderate-to-severe psoriasis in the DERMBIO registry treated with reference etanercept or switched from reference etanercept to etanercept biosimilar SB4 (Citation2).
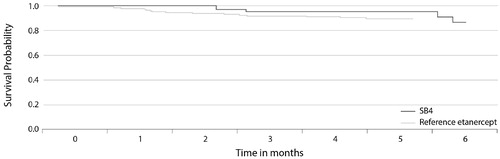
Table 1. Biosimilars with EMA approval for the treatment of moderate-to-severe psoriasis (current October 2018) (Citation1, Citation58, Citation59).
