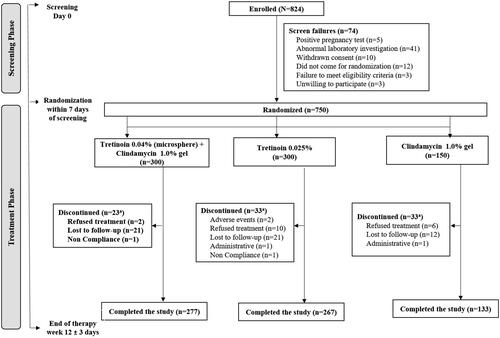
Figures & data
Table 1. Patient baseline demographics and clinical characteristics by treatment groups (ITT population).
Figure 2. Comparison of mean percentage change in inflammatory, non-inflammatory and total lesion counts between treatment groups (ITT analysis set). †††p < .001 combination vs. clindamycin monotherapy; *p < .05, **p < .01 combination vs. tretinoin monotherapy. ITT: intent-to-treat.
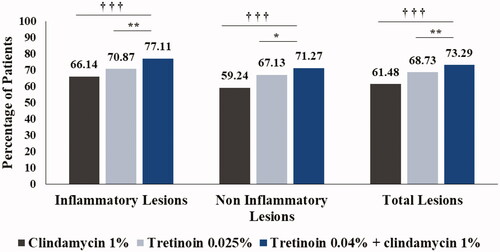
Table 2. Primary and secondary efficacy endpoints (ITT population).
Figure 3. Comparison of physician’s global evaluation of efficacy (ITT analysis set). ITT: intent-to-treat.
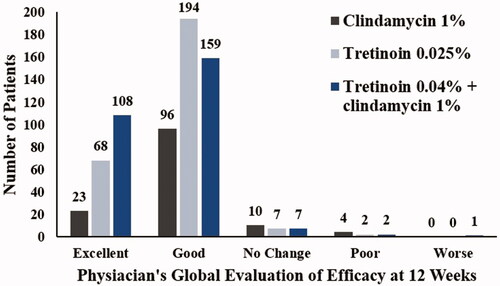
Table 3. Summary of adverse events.