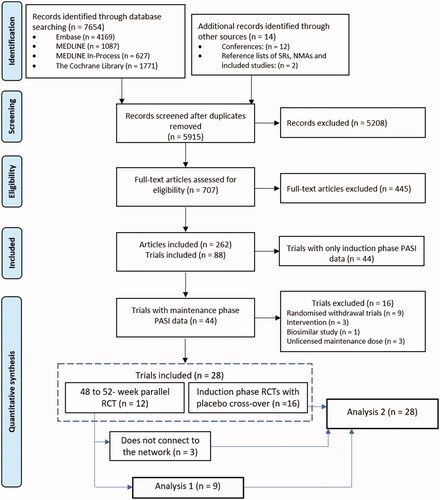
Figures & data
Table 1. Baseline characteristics of studies included in the primary analysis and the secondary analysis.
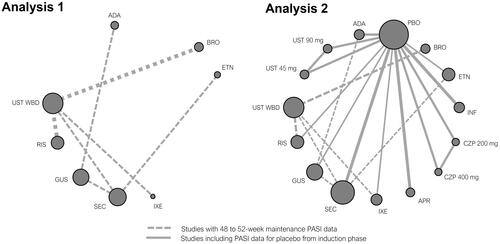
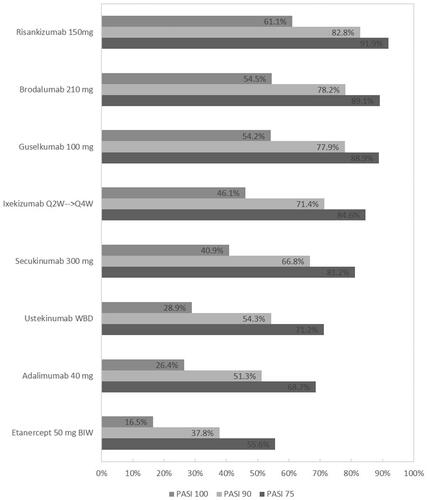
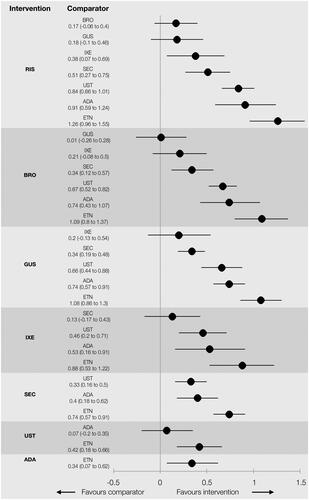
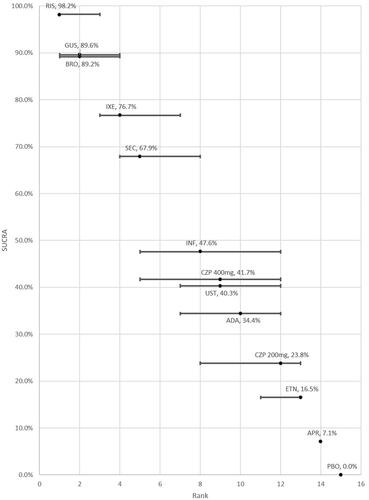
Table 2. NMA of 52-week active therapy RCTs (Analysis 1): results for PASI response.
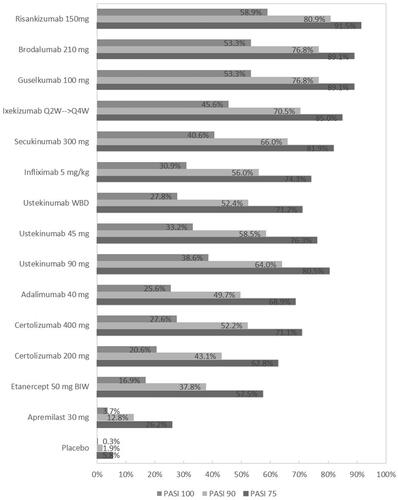
Table 3. NMA of 52-week RCTs using induction phase placebo control (Analysis 2): results for PASI responses.