Figures & data
Figure 1. Study population for post hoc analysis. ETN: etanercept; PBO: placebo; SEC: secukinumab. aOne patient from each study was excluded from the pooled analysis as a result of protocol deviations.
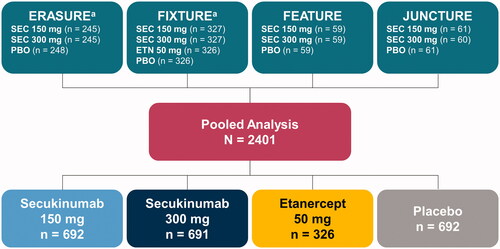
Table 1. Patients with active baseline comorbidities of psoriasis pooled from the ERASURE, FIXTURE, FEATURE, and JUNCTURE studies (full-analysis set).
Table 2. Baseline demographics, treatment histories, and disease characteristics of patients with psoriasis stratified by presence vs. absence of active comorbiditiesa.
Table 3. Relationships between comorbidities and baseline demographics and treatment histories of patients with psoriasis pooled from the ERASURE, FIXTURE, FEATURE, and JUNCTURE studies.
Figure 2. Efficacy as measured by (A) PASI75, (B) PASI90, and (C) PASI100 among patients with psoriasis stratified by presence or absence of active baseline comorbidities. PASI: Psoriasis Area and Severity Index. *p<.05 compared with etanercept and placebo. **p<.05 compared with secukinumab 150 mg, etanercept, and placebo.
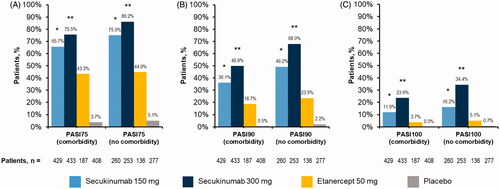
Figure 3. Efficacy as measured by (A) IGA mod 2011 0/1 or (B) IGA mod 2011 0 among patients with psoriasis stratified by presence or absence of active baseline comorbidities. IGA mod 2011, Investigator’s Global Assessment modified 2011. *p<.05 compared with etanercept and placebo. **p<.05 compared with secukinumab 150 mg, etanercept, and placebo.
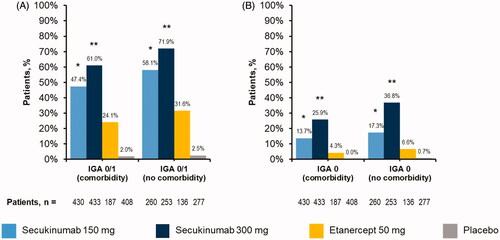
Table 4. Relationships between treatment response and baseline covariates across all patients with psoriasis pooled from the ERASURE, FIXTURE, FEATURE, and JUNCTURE studiesa.