Figures & data
Table 1. Summary of patient-reported outcomes (BREEZE-AD1 and BREEZE-AD2) at week 16.
Figure 1. DLQI total score ≤5 response rate in BREEZE-AD1 (a) and in BREEZE-AD2 (b). DLQI: Dermatology Life Quality Index; N: number of patients in the analysis population; NRS: Numeric Rating Scale. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo. For continuous endpoints, LS means are from MMRM analyses. For categorical endpoints, a nonresponder imputation was applied at censoring.
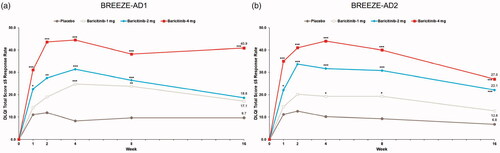
Figure 2. Percent change from baseline in Itch NRS severity in BREEZE-AD1 (a) and BREEZE-AD2 (b). N: number of patients in the analysis population; NRS: Numeric Rating Scale. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo. For continuous endpoints, LS means are from MMRM analyses. For categorical endpoints, a non-responder imputation was applied at censoring.
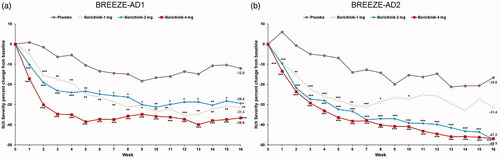
Figure 3. Percent change from baseline in SCORAD Itch in BREEZE-AD1 (a) and in BREEZE-AD2 (b). SCORAD: SCORing Atopic Dermatitis. Data reported as % change in LS means from MMRM analyses. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
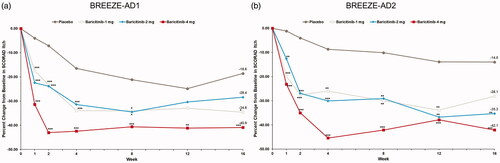
Figure 4. Percent change from baseline in POEM Itch in BREEZE-AD1 (a) and in BREEZE-AD2 (b). POEM: Patient Oriented Eczema Measure. Data reported as % change in LS means from MMRM analyses. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
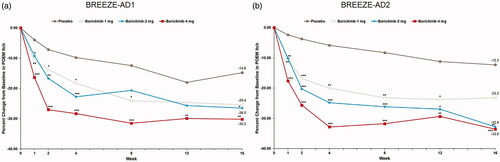
Figure 5. Percent change from baseline in Skin Pain NRS in BREEZE-AD1 (a) and in BREEZE-AD2 (b). NRS: Numeric Rating Scale. Data reported as % change based on the (CFB LSM from MMRM)×100/(pooled baseline mean). *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
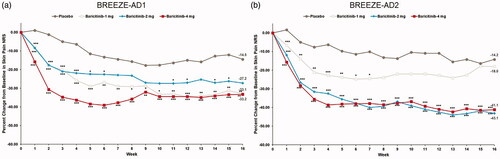
Figure 6. Skin Pain NRS ≥4 improvement response rate in BREEZE-AD1 (a) and in BREEZE-AD2 (b). NRS: Numeric Rating Scale. Primary censoring rule excludes data collected after first rescue therapy date or permanent study drug discontinuation. p Values obtained by logistic regression analysis with treatment, baseline value, region and baseline disease severity (IGA) as factors. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
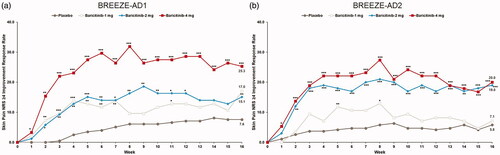
Figure 7. Percent change from baseline in SCORAD Sleep Loss in BREEZE-AD1 (a) and in BREEZE-AD2 (b). SCORAD: SCORing Atopic Dermatitis. Data reported as % change in LS means from MMRM analyses. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
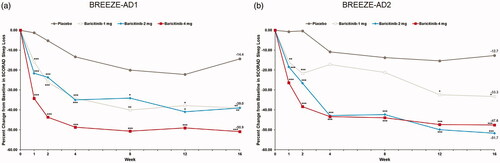
Figure 8. Percent change from baseline in POEM Sleep Loss in BREEZE-AD1 (a) and in BREEZE-AD2 (b). POEM: Patient Oriented Eczema Measure. Data reported as % change in LS means from MMRM analyses. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
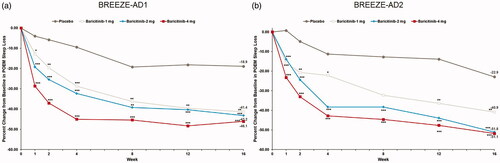
Table 2. Correlation between changes from baseline at week 4 and week 16 in Itch NRS, Skin Pain NRS, ADSS Item 2, and EASI (LOCF) and changes from baseline at week 4 and week 16 in PGI-S-AD and DLQI (LOCF) and by baricitinib treatmenta.
Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
