Figures & data
Figure 1. Flow chart CONDOR Extension study, Controlled Dose Reduction of Biologics with 24 months follow up.
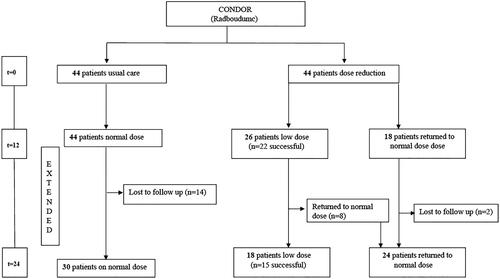
Table 1. Patient characteristics condor extension.
Figure 2. PASI and DLQI. (A) Psoriasis Area and Severity Index (PASI) comparison between usual care and dose reduction. PASI scores (median and IQR) are depicted for the original CONDOR study (month 0, 3, 6, 9 and 12) and for the CONDOR extension phase (month 15, 18, 21, 24). Significant differences were seen at 9 months (p = .005), at 12 months (p = .001), at 15 months (p = .002) and at 18 months (p = .003). (B) Dermatology Quality and Life Index (DLQI) depicted for the original CONDOR study (month 0, 3, 6, 9 and 12) and for the CONDOR extension phase (month 15, 18, 21, 24). Significant differences between dose reduction and usual care were only observed at 6 months (p = .005). No significant differences were observed in the extension period. *Significant differences that were observed between dose reduction and usual care.
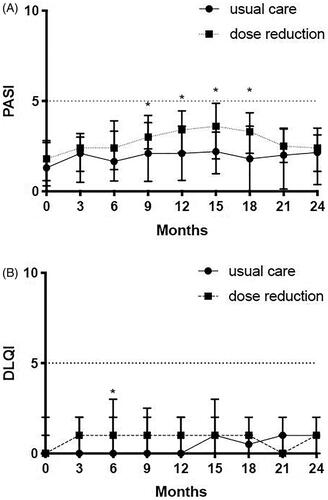
Table 2. Safety during condor extension (t = 12 to 24 months).
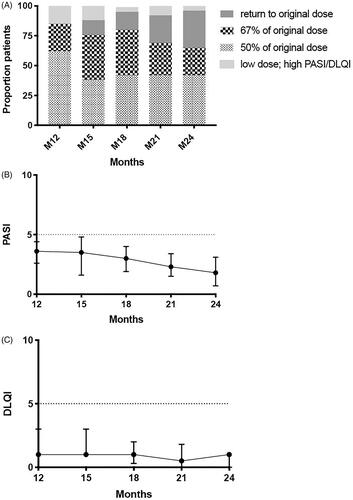
Figure 3. Dose, PASI and DLQI of 26 patients that started the CONDOR extension phase on a low dose (1-year extension phase). (A) Proportions of patients with successful dose reduction or failure, and their specific dosages. (B) Psoriasis Area and Severity Index (PASI) course (medians with interquartile ranges (IQR)). Missing M12 = 0, M15 = 2, M18 = 2, M21 = 2 and M24 = 1. 3. (C) Dermatology Quality and Life Index (DLQI) course (medians with IQR). Missing M12 = 0, M15 = 3, M18 = 2, M21 = 2 and M24 = 3.
Supplemental Material
Download PDF (345.3 KB)Data availability statement
All data will be shared with restrictions upon reasonable request when legally and ethically permitted by Dutch law.
Trial registration: The CONDOR study was registered at ClinicalTrials.gov, NCT 02602925.
