Figures & data
Table 1. Baseline demographic and clinical characteristics, overall and by itch and sleep improvement subgroups.
Figure 1. Proportion of patients with and without itch improvement achieving DLQI endpoints at Week 16. AD: atopic dermatitis; DLQI: Dermatology Life Quality Index. ***p < .0001. Itch improvement is defined as a ≥ 4-point decrease in the Itch Numeric Rating Scale at Week 16.
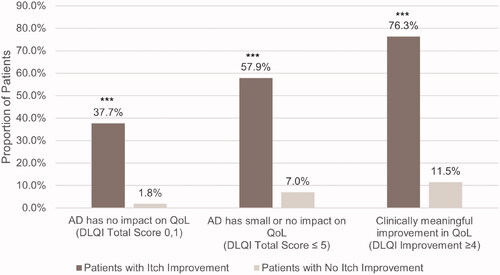
Figure 2. Mean change from baseline in work productivity and daily activity impairment in patients with and without itch improvement. Scores are from the Work Productivity and Activity Impairment Questionnaire – Atopic Dermatitis. ***p ≤ .0001. Itch improvement is defined as a ≥ 4-point decrease in the Itch Numeric Rating Scale at Week 16. Absenteeism, presenteeism, and overall work impairment were measured in employed patients only (n = 253).
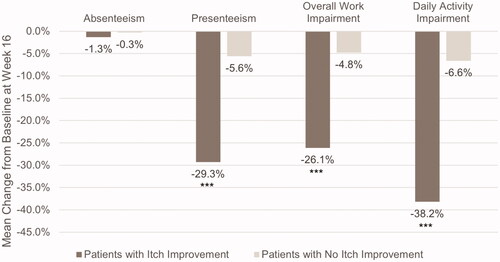
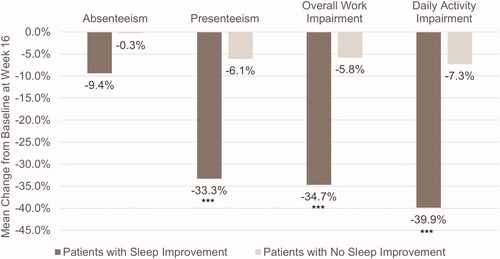
Figure 3. Proportion of patients with and without sleep improvement achieving DLQI endpoints at Week 16. AD, atopic dermatitis; DLQI, Dermatology Life Quality Index. ***p < .0001. Sleep improvement is defined as a ≥ 1.5-point decrease in Atopic Dermatitis Sleep Scale Item 2 score at Week 16.
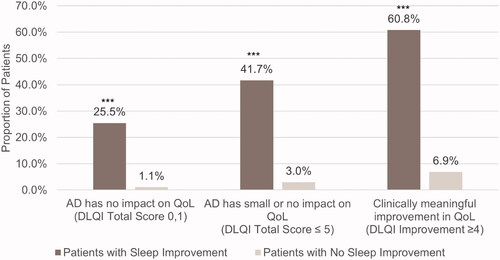
Figure 4. Mean change from baseline in work productivity and daily activity impairment in patients with and without sleep improvement. Scores are from the Work Productivity and Activity Impairment Questionnaire – Atopic Dermatitis. ***p < .0001. Sleep improvement is defined as a ≥ 1.5-point decrease in Atopic Dermatitis Sleep Scale Item 2 score at Week 16. Absenteeism, presenteeism, and overall work impairment were measured in employed patients only (n = 148).