Figures & data
Figure 1. Maintenance or gain of treatment success 4 weeks posttreatment with HP/TAZ lotion (pooled phase 3 studies). Treatment success was defined as ≥2-grade improvement from baseline IGA and a score of 0 (clear) or 1 (almost clear). Data shown for Week 12 treatment success, stratified by participants with or without week 8 treatment success. HP/TAZ: halobetasol propionate 0.01% and tazarotene 0.045%; IGA: Investigator’s Global Assessment.
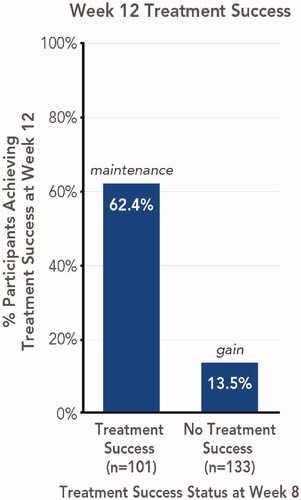
Figure 2. Posttreatment maintenance of treatment success with HP/TAZ lotion. Treatment success was defined as ≥2-grade improvement from baseline IGA and a score of 0 (clear) or 1 (almost clear). Representative images from a participant with target lesion on the lower back. Participant IGA: baseline, 3; weeks 8 and 12, 1. Participant BSA: baseline, 11%; week 8, 11%; week 12, 3%. BSA: body surface area; HP/TAZ: halobetasol propionate 0.01% and tazarotene 0.045%; IGA: Investigator’s Global Assessment.
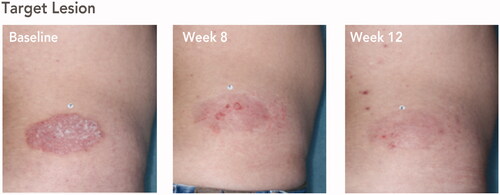
Figure 3. Posttreatment gain of treatment success with HP/TAZ lotion. Treatment success was defined as ≥2-grade improvement from baseline IGA and a score of 0 (clear) or 1 (almost clear). Representative images from a participant with target and non-target lesions on the lower leg. aLesions indicated by white stickers on participant’s leg; target lesion indicated by sticker with letter ‘T’. Participant IGA: baseline, 3; week 8, 2; week 12, 0. Participant BSA: baseline, 6%; week 8, 4%; week 12, 0%. BSA: body surface area; HP/TAZ: halobetasol propionate 0.01% and tazarotene 0.045%; IGA: Investigator’s Global Assessment.
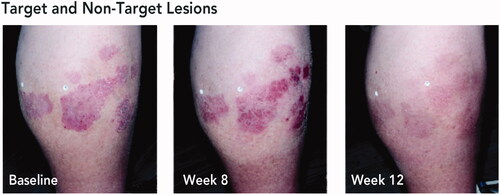
Figure 4. Posttreatment IGA severity by week 8 results (pooled phase 3 studies). aRemained severe at week 12. IGA assessed on a 5-point scale: 0 = clear, 1 = almost clear; 2 = mild; 3 = moderate; 4 = severe. Treatment success defined as ≥2-grade improvement from baseline IGA and a score of 0 (clear) or 1 (almost clear). IGA: Investigator’s Global Assessment.
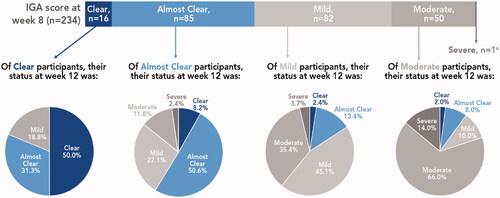
Figure 5. Signs of psoriasis maintenance or gain of treatment success 4 weeks posttreatment with HP/TAZ lotion (pooled phase 3 studies). Treatment success was defined as ≥2-grade improvement from baseline in individual signs of psoriasis at the target lesion. Data shown for Week 12 success, stratified by participants with or without week 8 success. HP/TAZ: halobetasol propionate 0.01% and tazarotene 0.045%; IGA: Investigator’s Global Assessment.
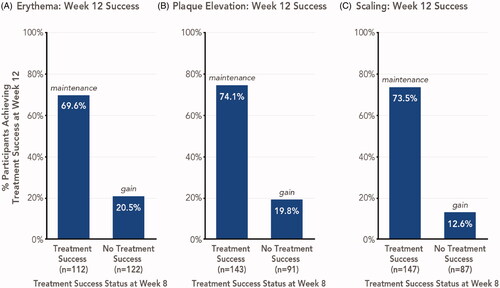
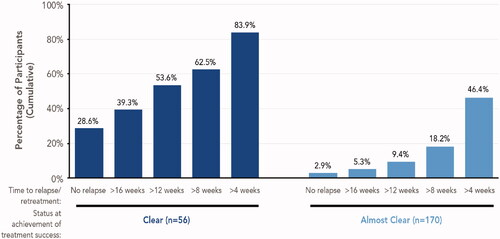
Figure 6. Maintenance of treatment success (long-term open-label study). Data shown for participants enrolled at least 8 weeks in the study and who stopped therapy after achieving treatment success, defined as an IGA score of 0 (clear) or 1 (almost clear). Cumulative data shown; within each subgroup, participants included in each bar are not mutually exclusive. Number of weeks to retreatment based on a 7-day week: 4 weeks = 28 days; 8 weeks = 56 days; 12 weeks = 84 days; 16 weeks = 112 days. IGA: Investigator’s Global Assessment.