Figures & data
Figure 1. Patient disposition and study drug discontinuation in (A) study 1 and (B) study 2. AI: autoinjector; PFS: prefilled syringe.
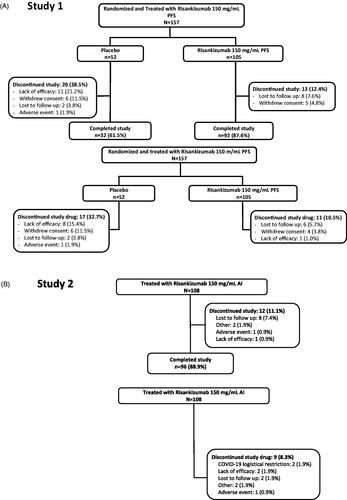
Table 1. Patient demographics and baseline disease characteristics in study 1 and 2.
Figure 2. Proportion of patients achieving PASI 90, PASI 100, sPGA 0/1, and sPGA 0 at week 16 with risankizumab and placebo (study 1) and OL risankizumab (study 2). NRI analysis. AI: autoinjector; NRI: non-responder imputation; OL: open-label; PASI: Psoriasis Area Severity Index; PFS: prefilled syringe; sPGA: static physician’s global assessment.
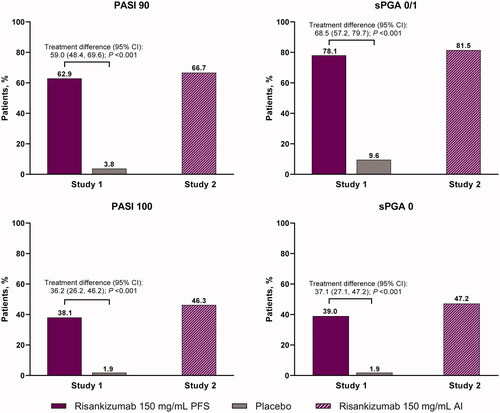
Table 2. Percentage change from baseline in PASI over time.
Figure 3. Mean SIAQ baseline PRE module domain scores and week 16 POST module domain scores and SIAQ POST module domain scores over time with risankizumab and placebo (study 1) and OL risankizumab (study 2). Observed case analysis. AI: autoinjector; BL: baseline; OL: open-label; PFS: prefilled syringe; SIAQ: Self-Injection Assessment Questionnaire.
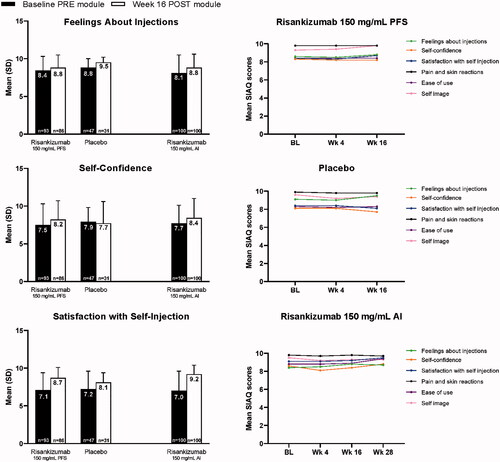
Table 3. Treatment-emergent adverse events in study 1 and 2.
Supplemental Material
Download PDF (379.8 KB)Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g. protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
