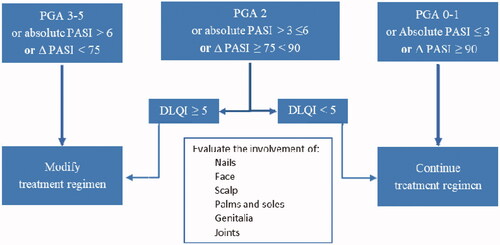
Figures & data
Table 1. Laboratory screening prior to initiation of a biologic treatment (Citation10,Citation12,Citation22–34).
Table 2. Screening for comorbidities (Citation10,Citation12,Citation22–34).
Table 3. Biologic agents and biosimilars available for the treatment of psoriasis in Saudi Arabia.
Table 4. Dosing schemes, type of antibody, efficacy, and half-life of biologic agents/biosimilars available for the treatment of psoriasis in Saudi Arabia.
Table 5. Adverse event of biologic agents for the treatment of psoriasis.
Figure 2. Management algorithm for the application of biologic therapy for the management of psoriasis. The order or treatments does not indicate priority. *16 weeks for skin and 24 weeks for joint disease. **IL-12/23 agonist used for patients with peripheral arthritis only.
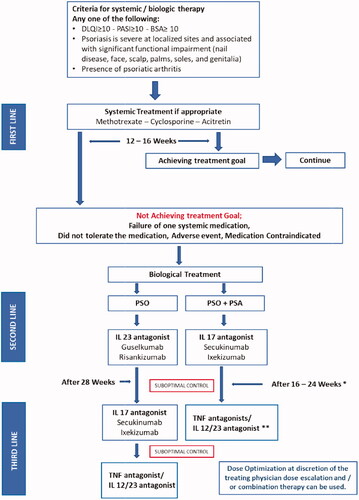
Table 6. Summary of recommendations for biologic therapy options in pregnancy.
Table 7. Summary of recommendations for choice of biologic therapy in pediatrics and adolescents.