Figures & data
Table 1. Patient demographics and baseline characteristics.
Figure 1. Time to first relapse during the maintenance phase with proactive management or reactive management with Cal/BD in the HPA subgroup. Cal/BD: calcipotriol 50 µg/g and betamethasone dipropionate 0.5 mg/g; HPA: hypothalamic-pituitary-adrenal. Censoring is not displayed for clarity.
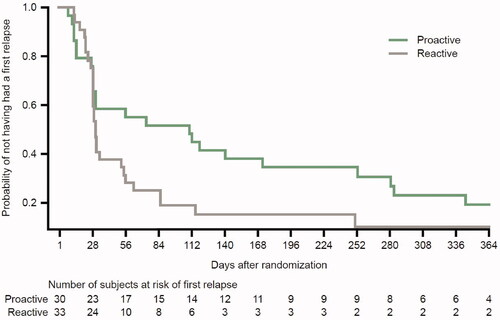
Table 2. Number of relapses and time in remission: summary.
Figure 2. Mean DLQI score by time during the maintenance phase for proactive and reactive Cal/BD management groups in the HPA subgroup and FAS. Cal/BD: calcipotriol 50 µg/g and betamethasone dipropionate 0.5 mg/g; DLQI: Dermatology Life Quality Index; FAS: full analysis set; HPA: hypothalamic-pituitary-adrenal; OL: open-label.
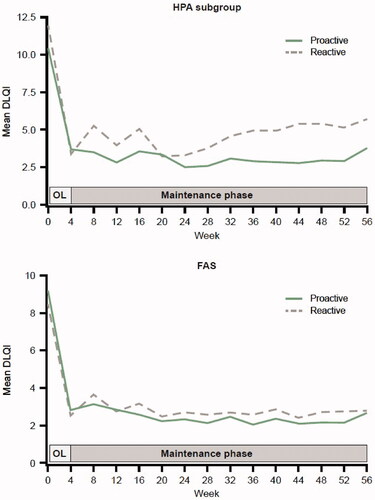
Table 3. Summary of AEs during the maintenance phase for the HPA subgroup.
Table 4. Treatment-related AEs during the maintenance phase for the HPA subgroup.
Table 5. Effect on corticosteroid metabolism.
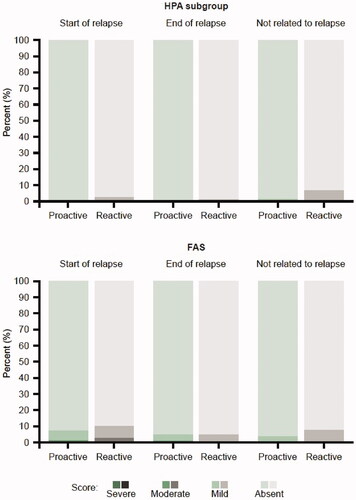
Figure 3. Patient’s perilesional assessment of local safety and tolerability (redness, dryness, edema, and erosion) relative to relapse in the HPA subgroup and FAS. Percentages are taken out of the total number of assessments done at visits where a relapse has started, a relapse has ended or where a relapse has not started or ongoing (not related to relapse). FAS: full analysis set; HPA: hypothalamic-pituitary-adrenal.