Figures & data
Table 1. Baseline demographics and disease characteristics from ECLIPSE study (Citation3).
Figure 1. Proportion of patients achieving at least a 90% improvement in Psoriasis Area and Severity Index (PASI 90) or PASI 100 response at week 48 by baseline age. 95% CI: 95% confidence interval; yrs: years.
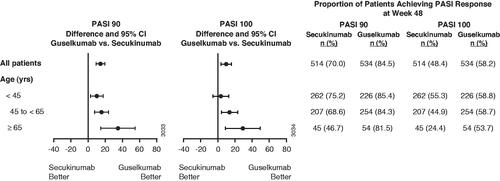
Figure 2. Proportion of patients achieving (a) at least a 90% improvement in Psoriasis Area and Severity Index (PASI 90) or (b) PASI 100 response at week 48 by baseline body weight deciles. kg: kilograms.
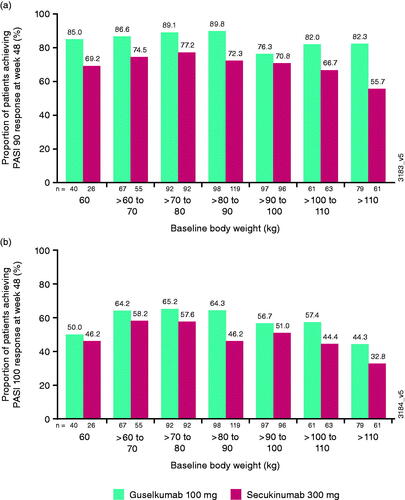
Figure 3. Proportion of patients achieving at least a 90% improvement in Psoriasis Area and Severity Index (PASI 90) or PASI 100 response at week 48 by baseline disease severity. 95% CI: 95% confidence interval; BSA: body surface area; IGA: Investigator’s Global Assessment; yrs: years.
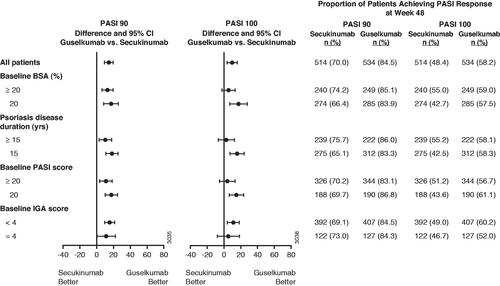
Table 2. Efficacy of Psoriasis Area and Severity Index component responses by body region at week 48.
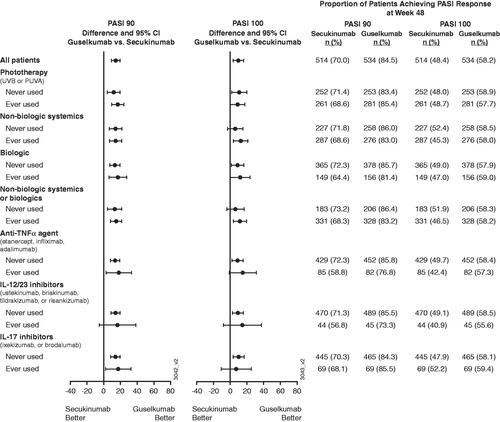
Figure 4. Proportion of patients achieving at least a 90% improvement in Psoriasis Area and Severity Index (PASI 90) or PASI 100 response at week 48 by prior psoriasis medication history at baseline. 95% CI: 95% confidence interval; IL: interleukin; PUVA: psoralen plus ultraviolet A; TNFα: tumor necrosis factor alpha; UVB: ultraviolet B.
Data availability statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
