Figures & data
Figure 1. Patient flow. The analysis is based on a cohort drawn from the German Psoriasis Registry PsoBest including 444 patients who had received fumaric acid esters (FAEs) treatment and met a minimal observation time of 12 months in the registry. *10–14 months after inclusion.
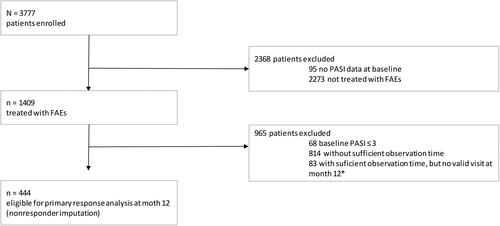
Table 1. Baseline demographics and disease characteristics.
Figure 2. Therapeutic effectiveness of fumaric acid esters (FAEs) at 12 months. Therapeutic response to FAEs treatment was defined as Psoriasis Area and Severity Index (PASI) ≤ 3 at 12 months or a treatment stop due to skin clearance. Nonresponder imputation (n = 444) and observed cases (n = 321) is shown.
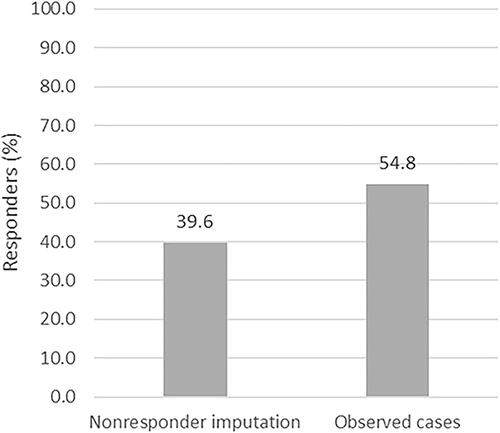
Table 2. Treatment effect modifiers of response to fumaric acid esters (FAEs).
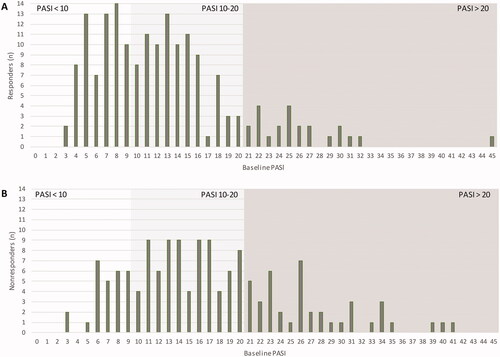
Figure 3. Distribution of Psoriasis Area and Severity Index (PASI) baseline scores in responders (A) and nonresponders (B) at 12 months. Patients eligible for response analysis had a minimum PASI > 3 at baseline. Therapeutic response to FAEs treatment was defined as PASI ≤ 3 at 12 months or a treatment stop due to skin clearance. Responders (n = 176), nonresponders (n = 145). Presented data are rounded.
Supplemental Material
Download MS Excel (14.3 KB)Data availability statement
All data generated or analyzed during this study are included in the published article.
