Figures & data
Table 1. Baseline demographics and disease characteristics in the adolescent mITT population.
Figure 1. Adolescent time-course response for IGA (0, 1) with ≥2-point improvement from baseline.
Percentage of patients (%) with IGA (0, 1) and ≥2-point reduction from baseline to Week 16 in the ADvocate (A) and ADhere (B) studies. *p < 0.05, **p < 0.01, ***p < 0.001, ap = 0.104 vs PBO using the Cochran-Mantel-Haenszel test adjusted by study (only for pooled ADvocate1 and ADvocate2), geographic region, and disease severity. IGA = Investigator’s Global Assessment; LEB = lebrikizumab; PBO = placebo; Q2W = every 2 weeks; TCS = topical corticosteroids.
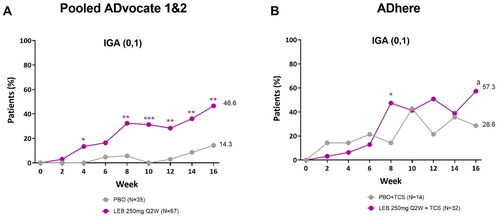
Figure 2. Adolescent time-course response for EASI clinical outcomes.
Percentage of patients (%) achieving EASI 75 in ADvocate (A) and ADhere (B) from baseline to Week 16; percentage of patients (%) achieving EASI 90 in ADvocate (C) and ADhere (D) from baseline to Week 16; EASI percentage change from baseline through 16 weeks in ADvocate (E) and ADhere (F). *p < 0.05, **p < 0.01, ***p < 0.001, ap = 0.113 vs PBO using the Cochran-Mantel-Haenszel test adjusted by study (for pooled ADvocate1 and ADvocate2 only), geographic region, and disease severity. EASI 75 = 75% improvement from baseline in Eczema Area and Severity Index score; EASI 90 = 90% improvement from baseline in Eczema Area and Severity Index score; LEB = lebrikizumab; LSM = least squares mean; PBO = placebo; Q2W = every 2 weeks; TCS = topical corticosteroids.
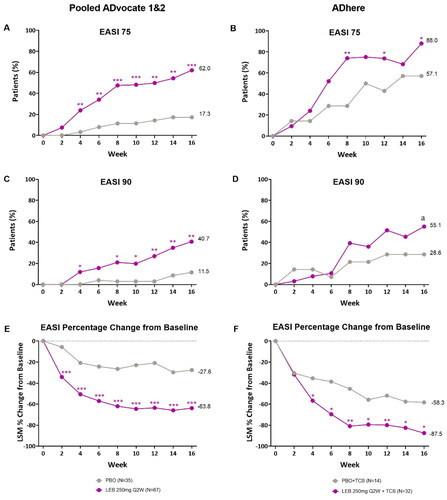
Figure 3. Adolescent time-course response for pruritus NRS ≥4-point improvementa.
Percentage of patients with a Pruritus NRS score of ≥4 points at baseline (%) achieving Pruritus NRS ≥4-point improvement from baseline in the ADvocate (A) and ADhere (B) studies. *p < 0.05, **p < 0.01, bp = 0.088 vs PBO using the Cochran-Mantel-Haenszel test adjusted by study (for pooled ADvocate1 and ADvocate2 only), geographic region, and disease severity. LEB = lebrikizumab; NRS = Numeric Rating Scale; PBO = placebo; Q2W = every 2 weeks; TCS = topical corticosteroids.
aPatients with baseline Pruritus NRS score ≥4.
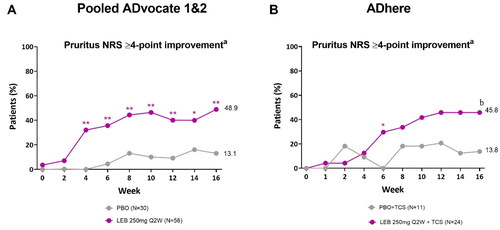
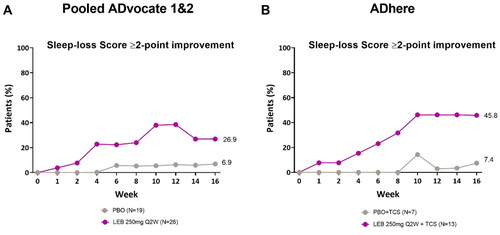
Figure 4. Adolescent time-course response for sleep-loss scale ≥2-point improvement.
Percentage of patients (%) with a Sleep-Loss score of ≥2 points at baseline achieving a ≥2-point improvement from baseline in the ADvocate (A) and ADhere (B) studies. The Cochran-Mantel-Haenszel test adjusted by study (for pooled ADvocate1 and ADvocate2 only), geographic region, and disease severity. LEB = lebrikizumab; PBO = placebo; Q2W = every 2 weeks; TCS = topical corticosteroids.
Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available for request 6 months after the indication studied has been approved in the United States and Europe and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
