Figures & data
Table 1. Demographic and clinical characteristics of patients who completed the survey.
Figure 1. CAL/BDP PAD-cream use in real-world according to (A) daily frequency, (B) application location, (C) length of use and (D) waiting time before dressing after application.
Rounded percentages calculated based on the total number of patients who completed the survey (n = 129).
aMean length of use was 14 weeks; bMean waiting time before dressing after application was 15 minutes.
CAL: calcipotriol; BDP: betamethasone dipropionate; PAD: polyaphron dispersion.
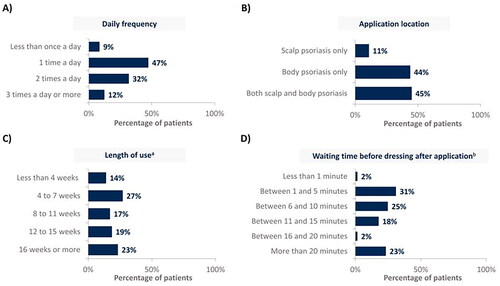
Figure 2. Percentage of patients who rated high agreement or complete agreement with each perception criterion, depending on whether it applied to CAL/BDP PAD-cream.
In the survey, each criterion could be rated by patients from 0 (‘do not agree at all’) to 10 (‘completely agree’). This figure only displays the percentage of patients who chose 8, 9, or 10 for each criterion. Rounded percentages calculated based on the total number of patients who completed the survey (n = 129).
CAL: calcipotriol; BDP: betamethasone dipropionate; PAD: polyaphron dispersion.
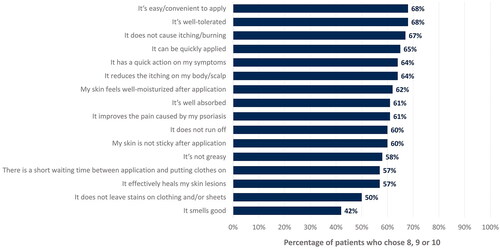
Figure 3. (A) Positive impact, (B) negative impact and (C) evaluation of CAL/BDP PAD-cream in a real-world setting.
Rounded percentages calculated based on the total number of patients who completed the survey (n = 129).
CAL: calcipotriol; BDP: betamethasone dipropionate; HCP: healthcare practitioner; PAD: polyaphron dispersion.
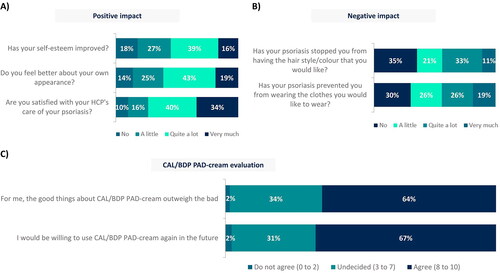
Figure 4. Comparison of CAL/BDP PAD-cream to the previous topical treatment used by patients.
Agree corresponds to overall positive responses (i.e., ‘agree’ and ‘strongly agree’). Disagree corresponds to overall negative responses (i.e., ‘disagree’ and ‘strongly disagree’). Rounded percentages calculated based on the total number of patients who had used a topical in the past (n = 67).
CAL: calcipotriol; BDP: betamethasone dipropionate; PAD: polyaphron dispersion.
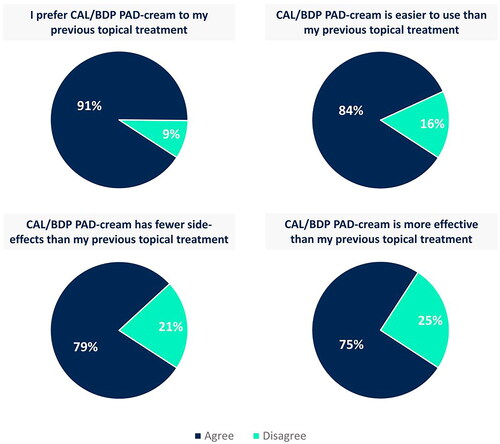
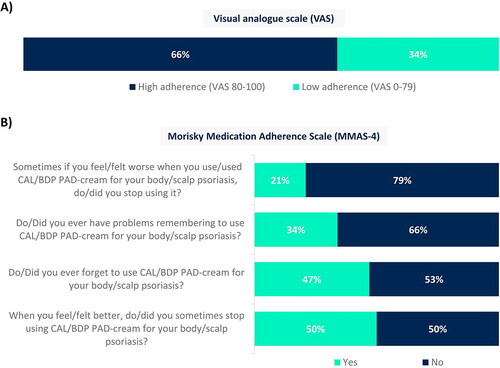
Figure 5. Adherence with CAL/BDP PAD-cream assessed through (A) a visual analogue scale (VAS) and (B) the Morisky Medication Adherence Scale (MMAS-4).
Rounded percentages calculated based on the total number of patients who completed the survey (n = 129).
CAL: calcipotriol; BDP: betamethasone dipropionate; MMAS-4: Morisky Medication Adherence Scale; PAD: polyaphron dispersion; VAS: visual analogue scale.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
