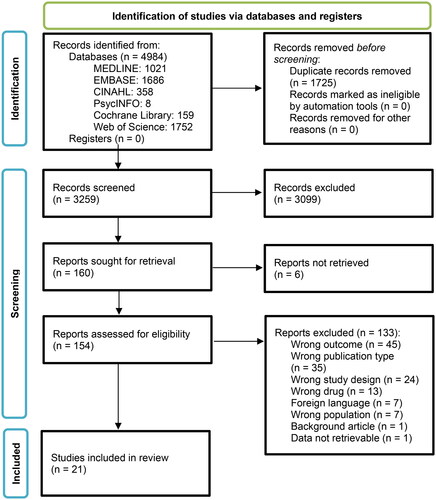
Figures & data
Table 1. Study characteristics.
Figure 2. Summary of risk of bias assessment for included studies using the Cochrane Collaboration tool.
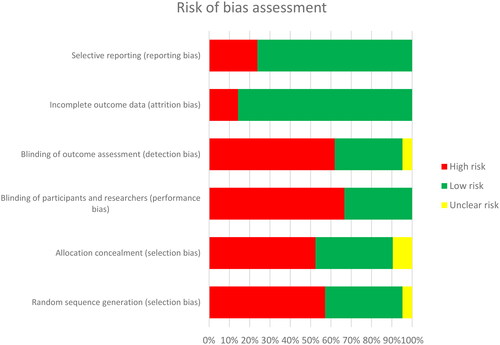
Figure 3. Number of outcome measurement instruments applied to assess itch, QoL or disease severity.
BEBSS: Birmingham Epidermolysis Bullosa Severity Score; DLQI: Dermatology Life Quality Index; EBDASI: Epidermolysis Bullosa Disease Activity and Scarring Index; GSS: global severity score; iscorEB: instrument for scoring clinical outcomes of research for epidermolysis bullosa; NRS: numerical rating scale; PedsQL: Pediatric Quality of Life Inventory; QOLEB: quality of life evaluation in epidermolysis bullosa; TBSA: total body surface area; VAS: visual analogue scale.
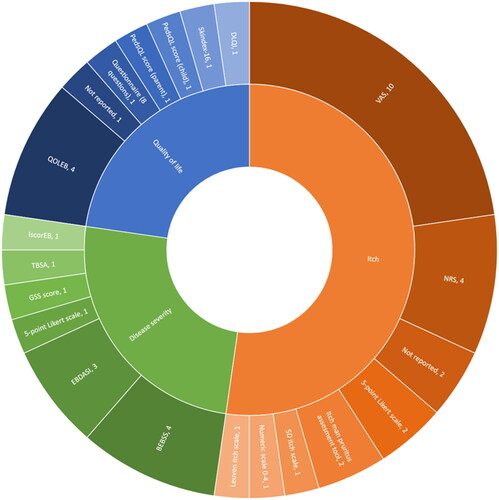
Table 2. Measurement instruments used to measure itch, QoL and disease severity.
Table 3. Overview of local and systemic treatment modalities reporting on itch outcomes for EB patients.
Table 4. Adverse events reported in clinical studies.
Supplemental Material
Download Zip (29.6 KB)Data availability statement
The data that support the findings of this review are available from the corresponding authors upon reasonable request.