Figures & data
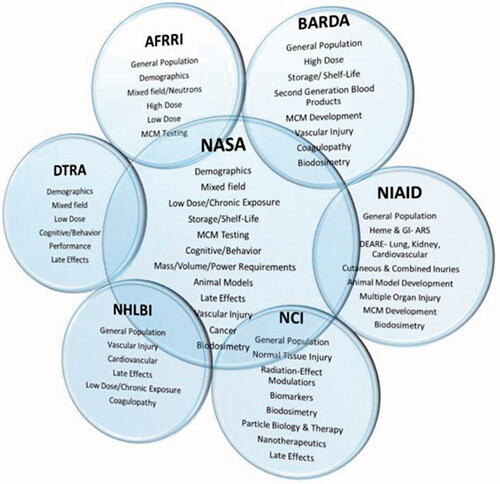
Figure 2. A suggested generalized workflow for the development of radioprotectors and mitigators for radiation oncology. The development of a radiation-effect modulator is a multi-step process from innovation to translation, as described in the illustration, which may involve the acquisition of intellectual property from an academic or industry source for development and translation to radiation oncology clinic following regulatory approval. The various steps in this workflow may include the following: sourcing of intellectual property, focusing development towards a solving a specific problem in the clinic, synthesis of the radiation-effect modulator, developing scientific evidence for organ/site-specific activity, evaluation of mechanism of action, formulation and dose/schedule optimization and performing studies on normal tissue toxicities, further evaluation in tumor bearing animals, if necessary, and conducting phase I, II, and III clinical trials. A close interaction among academia, small businesses, and clinical trial workgroups is crucial for successful translation of a radiation-effect modulator for ultimate use in the clinic (Prasanna et al. Citation2015). © 2019 Radiation Research Society. GLP: good laboratory practice; CRO: Contract Research Organization. NRG – NSABP: National Surgical Adjuvant Breast and Bowel Project; RTOG: the Radiation Therapy Oncology Group; GOG: the Gynecologic Oncology Group.
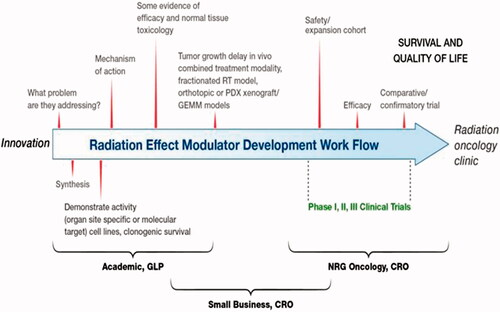
Figure 3. The NIAID/RNCP portfolio includes the evaluation of numerous MCMs and biodosimetry technologies. Shown here are the number of MCM and biodosimetry candidates evaluated based on RNCP funding source or program area (CMCRC: Centers for Medical Countermeasures Against Radiation Consortium; AFRRI: Armed Forces Radiobiology Research Institute, program grants, PDSS: Product Development Support Services contract; SBIR: Small Business Innovation Research grants, program contracts and other funding mechanisms). Candidates that may not have met the criteria for evaluation or were too early for consideration are labeled ‘Not Pursued.’ In addition, the number of candidates evaluated or not evaluated is further broken down into scientific areas of interest. Heme: hematopoietic; GI: gastrointestinal, lung, skin, cardiovascular, kidney; CNS: central nervous system, combined injury, radionuclide decontamination, biodosimetry.
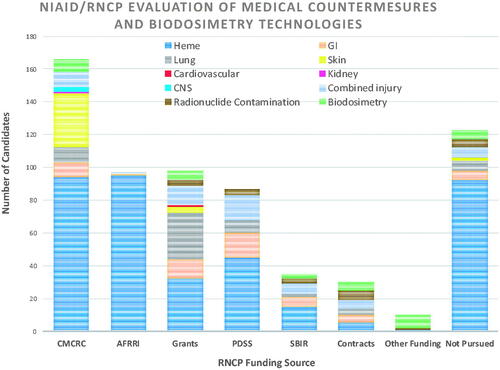
Figure 4. BARDA’s five focus areas for targeted product development include vascular injury, coagulopathies, inflammation, cell death, and ischemia.
Table 1. Medical countermeasure criteria for GCR radioprotection/mitigation.