Figures & data
Figure 1. The Kaplan–Meier curves and net radioprotection of 30-day survival of mice administered BIO 300 OP twice daily using various dose schedules. CD2F1 mice (n = 16/group) were administered (A) BIO 300 OP (200 mg/kg) or vehicle po, BID for 6 d prior to TBI or (B) BIO 300 IS (200 mg/kg) or its vehicle administered as a single im injection 24 h prior to TBI. (C) Net radioprotection of BIO 300 OP administered twice daily using various dose schedules or BIO 300 IS administered 24 h prior to TBI. Percent net radioprotection at 30 days was determined by subtracting BIO 300 treatment survival from the respective vehicle group’s survival for each dose schedule and BIO 300 formulation tested. All mice were irradiated with 9.2 Gy cobalt-60 (0.6 Gy/min, approximately LD70/30 dose). Survival was monitored for 30 d post-irradiation. Statistical significance for 30-day survival was determined by log-rank test (*p<.05).
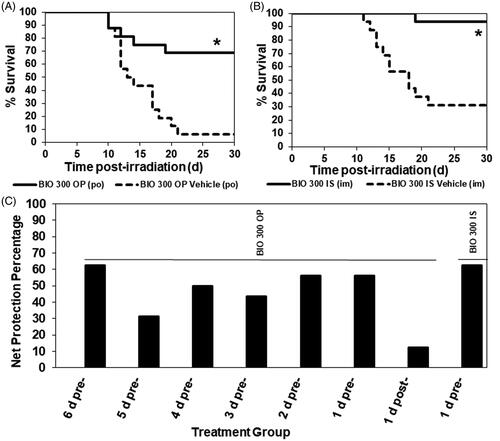
Table 1. Summary of BIO 300 oral powder 30-day dose scheduling study.
Figure 2. The Kaplan–Meier curves of 30-day survival of CD2F1 mice administered BIO 300 IS, OP, OS, or Neulasta. (A) Mice (n = 16/group) were administered BIO 300 IS (200 mg/kg) or its vehicle by im 24 h prior to TBI. BIO 300 OP (200 mg/kg) and BIO 300 OS (200 mg/kg) or their respective vehicle controls were administered BID, po to mice for 6 d prior to TBI. (B) Mice (n = 16/group) were administered Neulasta (300 µg/kg) sc or its vehicle 24 h post-TBI. All animals were irradiated with 9.2 Gy cobalt-60 (0.6 Gy/min, approximately LD70/30). Survival was monitored for 30 d post-TBI. *Statistical significance at day 30 between the drug treated group and respective vehicle control as determined by log-rank test (*p<.05, **p<.01, ***p<.001).
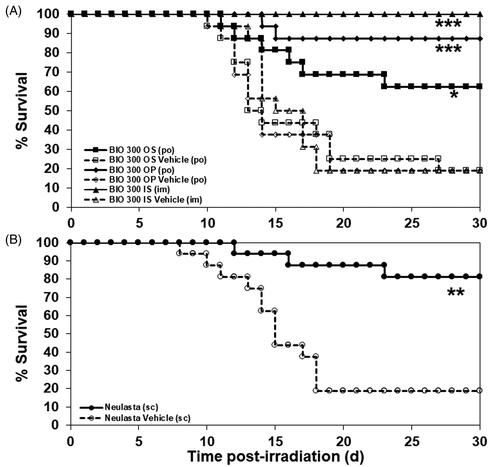
Figure 3. The Kaplan–Meier curves of 180-day survival of CD2F1 mice administered BIO 300 IS, OP, OS, or Neulasta. Mice that survived 30 d post-TBI in the above study were monitored for an additional 150 d until 180 d post-TBI. (A) Mice (n = 16/group) were administered the indicated BIO 300 formulation or vehicle as described above. (B) Mice (n = 16/group) were administered Neulasta or vehicle as described above. Survival was analyzed at 180 d post-TBI. *Statistical significance at day 180 between the drug treated group and respective vehicle control as determined by log-rank test (*p<.05, **p<.01, ***p<.001).
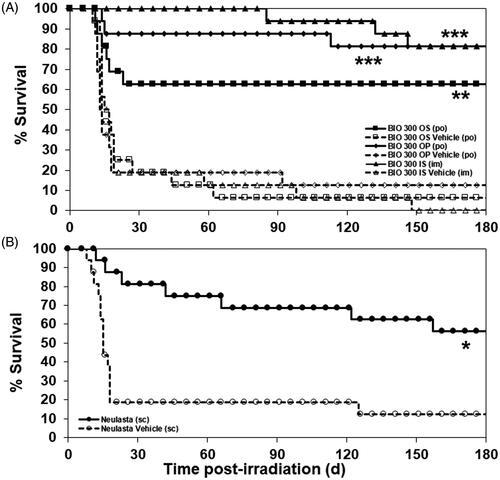
Table 2. Histopathological effects of total-body irradiation on lungs of CD2F1 male mice at day 180 after total-body irradiation.
Data availability statement
All data generated or analyzed during this study are included in this article.
