Figures & data
Table 1. Topic areas and sub-categories with statement selection by the steering group.
Figure 1. caption: Delphi study flow chart.
A flow chart explains the process of the Delphi study. An expert panel of 27 members was recruited, who participated in two rounds of the Delphi with controlled feedback after each round. A steering group of five members reviewed the results of each process. Ninety statements were initially generated and reviewed by the steering group and modified to 85 statements. These were distributed to the expert panel for round one. At the end of round, one 50 statements had consensus and 35 had no consensus. After steering group review 25 were submitted for round two. At the end of round two, a further 12 had consensus, six had majority agreement and seven had no consensus. After steering group review a decision was made to end the Delphi process. A total of 62 statements achieved consensus and six had majority agreement. These results formed the best practice recommendations and development of a swallow screening tool.
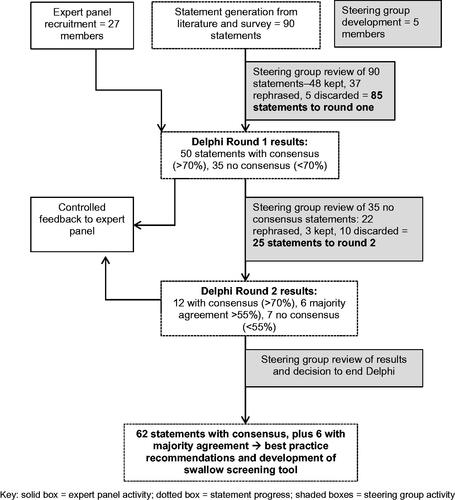
Table 2. Professionals invited and consented as expert panellists.
Table 3. Demographic details of expert panel participants.
Figure 2. caption: Number of statements per topic achieving consensus in Delphi round one.
A bar graph shows the results of the first Delphi round with the numbers of statements per topic with and without consensus. ‘Co-morbid status’ has seven statements with consensus and five with no consensus, ‘definition’ has nine with consensus and six with no consensus, ‘screening’ has eight with consensus and nine with no consensus, ‘assessment’ has two with consensus and three with no consensus, ‘identification’ has four with consensus and two with no consensus, ‘management’ has 13 with consensus and 10 with no consensus and ‘therapeutic management’ has seven with consensus and none with no consensus.
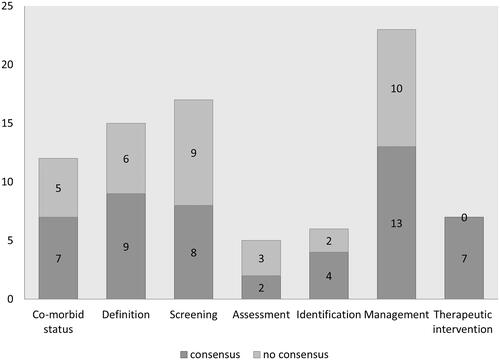
Table 4. Descriptive statistics and levels of consensus for round one statements.
Figure 3. caption: Number of statements per topic achieving consensus, majority agreement in Delphi round two.
A bar graph shows the results of the second Delphi round with the numbers of statements per topic with consensus, majority agreement or no consensus. ‘Co-morbid status’ has two statements with consensus, one with majority agreement and two with no consensus, ‘definition’ has four statements with consensus, none with majority agreement and two with no consensus, ‘screening’ has two with consensus, two with majority agreement and none with no consensus, ‘assessment’ has one with consensus, one with majority agreement and none with no consensus, ‘identification’ has none with consensus and one with no consensus, ‘management’ has three with consensus, two with majority agreement and two with no consensus and ‘therapeutic management’ has no statements in this round.
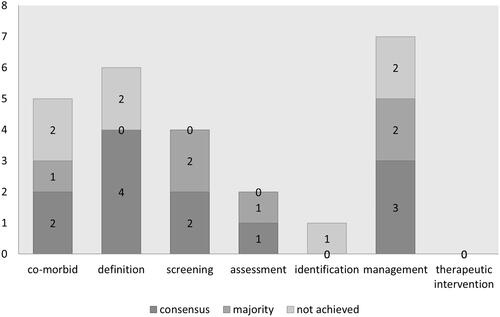
Table 5. Descriptive statistics for round 2 statements.
Table 6. Descriptive statistics for change in non-consensus statements between round 1 and round 2.
Table 7. Best practice recommendations for acute cSCI management of dysphagia and associated complications.
Table 8. Domains, category and sub-category of swallow risk screening tool.
Supplemental_file.pdf
Download PDF (219.6 KB)Data availability statement
All research data are stored securely in the Research Innovation Centre at the Royal National Orthopaedic Hospital ([email protected]).
