Figures & data
Table 1 . Culture strains employed in this study
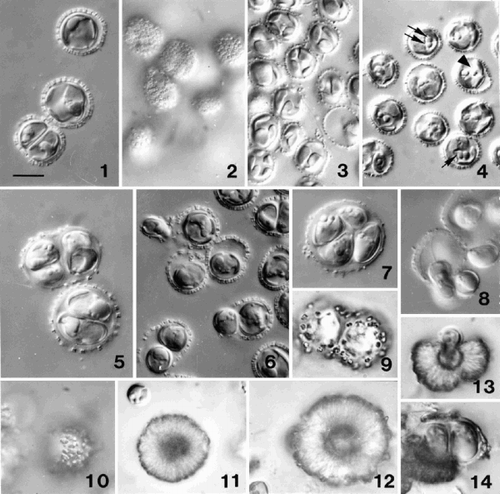
Figs 1–14. Light microscope (DIC) observations of Ochrosphaera neapolitana. . Optical section of non-motile cells showing coccoliths in profile. . Surface view of cells showing ring-shaped coccoliths in surface view. . Optical section showing thin lobed chloroplasts. . Cells fixed with osmium vapour revealing different aspects of the pyrenoid. Note the cell with separated pyrenoids (arrowhead), a pyrenoid with a long stalk (single arrow), and two pyrenoids abutting each other (double arrow). . Two and four cells in the same coccosphere in an older culture. The layer of scales thickens and the coccoliths become widely spaced as the coccosphere expands. . Division in a young culture showing one of the two cells being released from the coccosphere. The released cell is usually elongate (upper left) and possesses flagella. . Four cells in a coccosphere following vegetative division in an old culture. . Release of flagellated daughter cells from a coccosphere after immersion in new medium. –. Progressive stages in the over-calcification of coccoliths and the formation of pseudocysts. . Cultured cells after three months. . Surface view of a cell after several months in culture with individual over-calcified coccoliths still visible. . Intermediate stage in the formation of a pseudocyst (individual coccoliths no longer visible). . A heavily over-calcified pseudocyst. . Release of a naked cell from a pseudocyst. . Two cells within an envelope recently released from a pseudocyst (note newly formed coccoliths on envelope). Scale bar in represents 10 µm and applies to all figures.
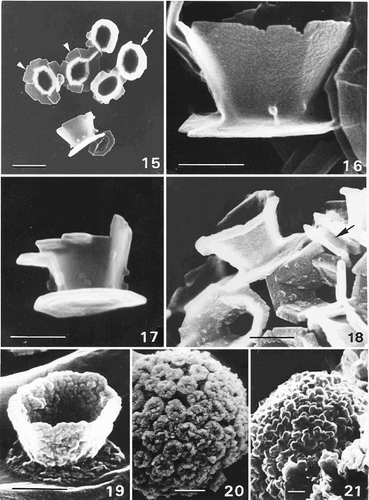
Figs 15–21. SEM observations of Ochrosphaera neapolitana coccoliths. . Group of coccoliths showing vase-shaped coccoliths with external protrusions in profile and from above (arrow) and two pulley-shaped coccoliths in surface view (arrowheads). . Detail of a vase-shaped coccolith in profile. . ‘Intermediate’ coccolith. . West's strain after one month of culture exhibiting small protrusions on the coccoliths. Pulley-shaped coccolith with a small distal flange (arrow). . Increasingly over-calcified coccolith. . Highly over-calcified coccosphere in which the central cavities of the coccoliths are almost filled. . Pseudocyst in which individual coccoliths are no longer recognisable. Scale bars represent 1 µm (), 0.5 µm (–) and 2 µm (, ).
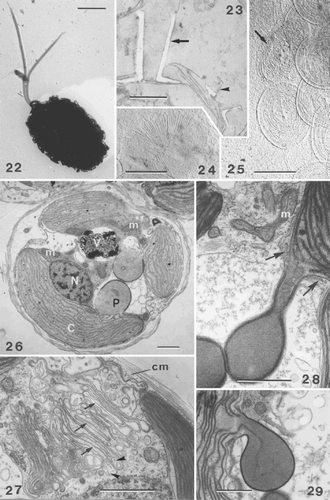
Figs 22–29. TEMs showing general cell ultrastructure and scale morphology of Ochrosphaera neapolitana. , , are shadowed with gold/palladium. . Coccolith-bearing flagellated cell. Note the absence of an emergent haptonema and the unequal acronematic flagella. . Section showing two extremes of coccolith shape, vase-shaped (arrow) and pulley-shaped (arrowhead), side by side on one cell. . Proximal face of a coccolith base-plate scale with radiating microfibrils in quadrants. . Distal face of coccolith base-plate scale (arrow) showing a vaguely concentric pattern of microfibrils. Circular body scales with an identical pattern of superficial, irregularly concentric microfibrils and a thin rim on each face are also visible. . Section of whole cell showing the main cell components: nucleus (N), chloroplasts (C), pyrenoid (P) (here with an enlarged base), mitochondrion (m), vacuole (V). . Detail of Golgi body showing several forming scales in the middle and distal parts of the Golgi stack (arrows). Columnar material (cm) is visible against the plasmalemma and in Golgi vesicles (arrowheads). . Stalked pyrenoid: arrows indicate the periplastidial reticulum at the base of the pyrenoid. . Pyrenoid thylakoid continuous with that of the chloroplast. Scale bars represent 2 µm (), 0.5 µm (–), and 1 µm (–).
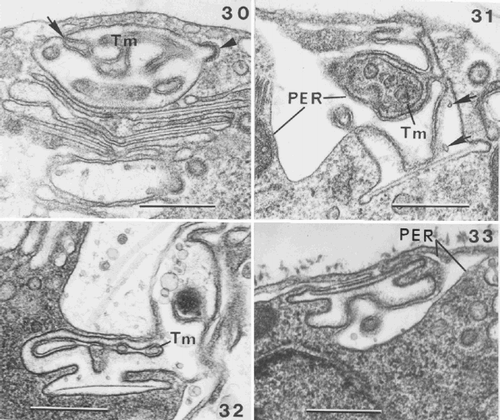
Figs 30–33. Coccolithogenesis in Ochrosphaera neapolitana. . Oblique section of the distal part of a coccolith vesicle showing the distal tubular matrix containing densely stained material (Tm) continuous with the PER (arrow). The internal membrane of the coccolith vesicle is lined with densely staining material and a coated vesicle fusing with the coccolith vesicle (arrowhead). . Section of a vesicle containing vase-shaped coccolith at an advanced stage of development, showing interconnections of the well developed tubular matrix (Tm) and PER with the coccolith vesicle. Arrows indicate points within the coccolith vesicle, which are presumed first stages of calcification (calcite presumably dissolved during fixation). , . Advanced stages in the formation of pulley-shaped coccoliths. . Portion of a cell showing that the lining of the coccolith vesicle is denser than the central clearer zone where calcification will occur. Note reduced tubular matrix (Tm). . Cell showing that the PER follows the contours of the coccolith vesicle. Note the different orientation of the coccolith vesicle in , . Scale bars represent 0.5 µm.
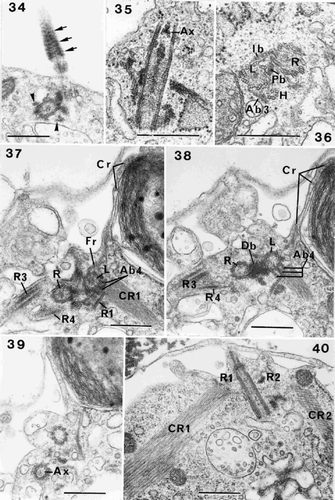
Figs 34–40. Flagellar root structure in Ochrosphaera neapolitana. . Tangential section of a flagellum showing the probable presence of the helical band at the base of the axoneme (arrow). Roots (R3, R4) are also visible in transverse section (arrowheads). . Longitudinal section of a flagellar base at the level of the axosome (Ax). . Transverse section of left and right flagellar bases (L and R) and haptonematal base (H) with five microtubules. Proximal and intermediary (Pb and Ib) striated bands connect the flagellar bases, and the accessory band links the haptonematal base and the left flagellar base (Ab3). –. Serial sections of the flagellar bases in transverse section: . Section through root (R1) showing linkage via accessory band (Ab4) to the left flagellar base. The crystalline component (CR1) is visible. Roots (R3, R4) extend from either side of the right flagellar base. The contractile root (Cr) extends along the surface of the chloroplast at the periphery of the cell; the connection of the Cr with the fibrous root (Fr) emanating from the left flagellar base is visible. . Section at the level of the Db. Accessory band (Ab4) is present. . Section through one of the flagella at the level of the axosome (Ax). . Section of roots (R1, R2) and their crystalline components (CR1, CR2). Scale bars represent 0.5 µm.
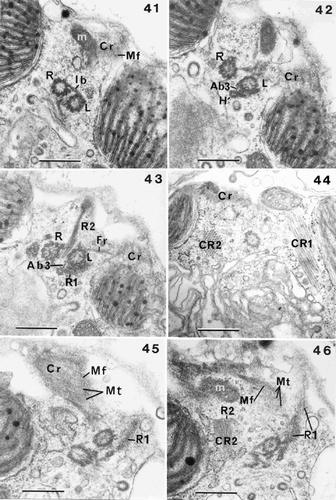
Figs 41–46. Flagellar root structure in Ochrosphaera neapolitana. –. Serial transverse sections of flagellar bases showing partial tangential views of the contractile root (Cr) with interconnecting microfilaments (Mf). . Section showing the right and left flagellar bases linked by the intermediary striated connecting band (Ib); a mitochondrion (m) is visible. . Section nearer the cell surface showing the haptonematal base (H) linked to the left flagellar base by a small non-striated accessory band (Ab3). . Section showing root (R1) in contact with the haptonematal base. Root (R2) shown in longitudinal section with the fibrous root (Fr) also visible. . Crystalline root (CR2) is seen in transverse section, CR1 in longitudinal section, and part of the Cr is also illustrated. , . Serial tangential sections through the sheet of microtubules (Mt) of the Cr. . Section through microtubules (Mt) of Cr, showing their regular spacing and linkage by microfilaments (Mf). . Section showing Cr in close proximity to a mitochondrion. Root (R2) and its crystalline component (CR2) (in longitudinal section) are also visible. Scale bars represent 0.5 µm.
Figs 47–48. Flagellar root structure in Ochrosphaera neapolitana. , . Serial sections of root (R1) and the microtubular plate, the crystalline root (CR1) and the contractile root (Cr). . Section through the junction of R1 with the fibrous root (Fr), and the localisation of the Cr under the plasmalemma close to the flagellar bases. . Section showing microfilaments joined to the microtubules after the fusion of R1 and Fr. R1 is sectioned transversely. Scale bars represent 0.5 µm.
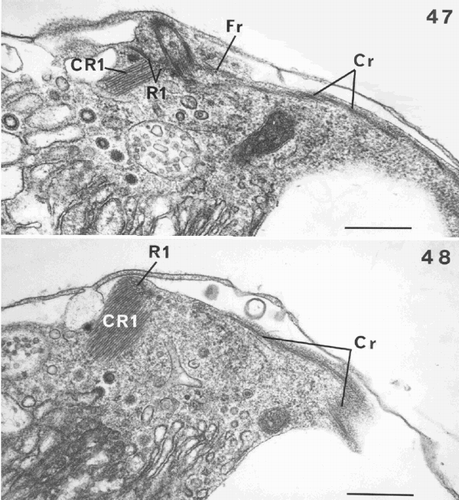
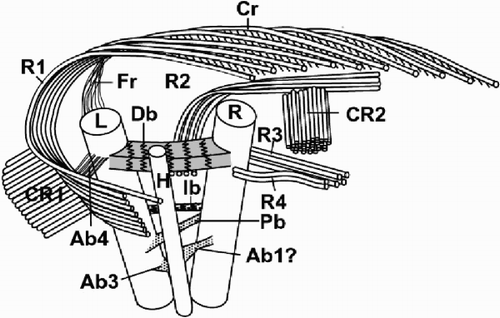
Fig. 49. Diagram showing the flagellar apparatus of Ochrosphaera neapolitana. The position of the accessory bands is approximate relative to the flagellar bases. Left and right flagellum bases (L, R); haptonematal base (H); roots (R1, R2, R3, R4); crystalline components (CR1, CR2); contractile root (Cr); fibrous root (Fr); accessory bands (Ab1, Ab3, Ab4); proximal, distal and intermediate bands (Pb, Db, Ib). Not drawn to scale.
Figs 50–57. Life-cycle of Ochrosphaera neapolitana. . Aspects of the S-cell generation. Cells are naked and have the same cytological characteristics as coccolith-bearing cells. . Mucilage coating surrounding S-cells highlighted by staining with ruthenium red. . Contrasting aspects of colonies of each generation grown on agar. C-cell colonies (arrow) are compact and dark in colour; S-cell colonies are more dispersed and lighter. . Tetrad (= meiocyst; compare with ) preceding the reappearance of S-cells. . Naked S-cells released from the meiocyst. . Scales characteristic of the S-cell generation. The most abundant scales are irregular in shape and cover the plasmalemma (basal scales). The proximal faces (bspf) have radial fibres in quadrants and their distal faces (bsdf) have concentric fibres. Both faces of the superficial scales are also seen. The proximal faces (sspf) of these scales have radiating fibres and a thin rim; the distal faces (ssdf) have concentric fibres that are difficult to resolve. , . Metaphase chromosomes fixed and stained with lactopropionic orcein. . Diploid C-cell with 20 ± 1 chromosomes. . Haploid S-cell with 10 ± 1 chromosomes. Scale bars represent 10 µm (, , , ), 50 µm (), 0.5 µm () and 5 µm (, ).
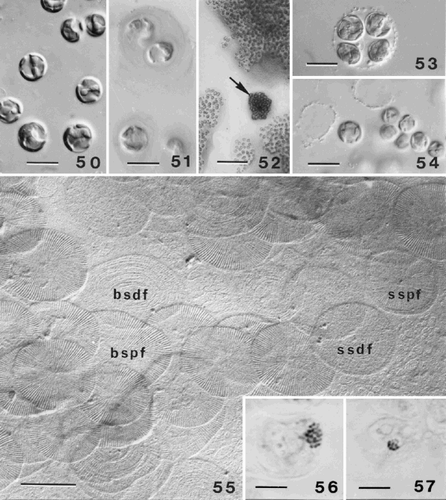
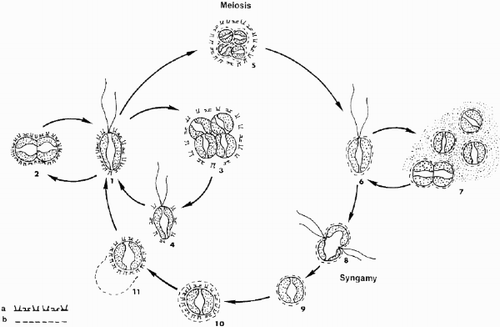
Fig. 58. Schematic representation of the presumed life cycle of Ochrosphaera neapolitana. (a) cellular coating of diploid coccolith-bearing stage; (b) cellular coating of haploid scaly stage. 1. Motile coccolith-bearing (C) cell; 2–4. vegetative division; 5. meiocyst; 6. motile scale-bearing (S) cell; 7. palmelloid S stage and vegetative division; 8. fusion; 9. zygote; 10. formation of C cell inside zygote envelope; 11. liberation of C cell. Note: stages 6, and 8 to 11 are hypothetical.