Figures & data
Table 1 . Slides and locations studied
Table 2 . Culture sources, voucher material and molecular sequence data
Table 3 . Character matrix of frustule, protoplast and reproductive characteristics of selected raphid species, for cladistic analysis.a Due to incomplete data for any particular species that might be used as a terminal taxon, the outgroup is a genus (Eunotia) with alternative states for two characters. The polymorphism of Rhoicosphenia curvata (Kützing) Grunow for auxospore caps may be genuine or may reflect different specimen preparation methods: Cholnoky (Citation1927) observed caps in stained material, but Geitler (Citation1952a) and Mann (Citation1982b) did not see them in live material
Table 4 . Frustule, protoplast and reproductive characteristics of selected genera of raphid diatoms (principally those included in the rbcL analysis) for comparison with Petroneis and Lyrella a
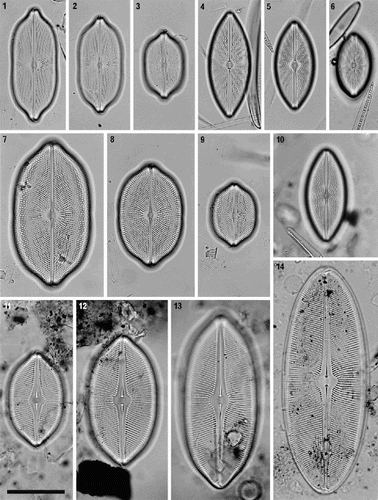
Figs 1–14. Petroneis species found in the British Isles. LMs of acid-cleaned material, showing size and shape variation in vegetative populations. –. P. humerosa. –. P. marina. –. P. monilifera. . P. granulata. Only one valve of this species was observed. –14. P. latissima, including a post-auxospore valve (), in which valve outline and pole shape are simpler than smaller valves in the size reduction cycle (–13). Scale bar (in ) represents 50 µm.
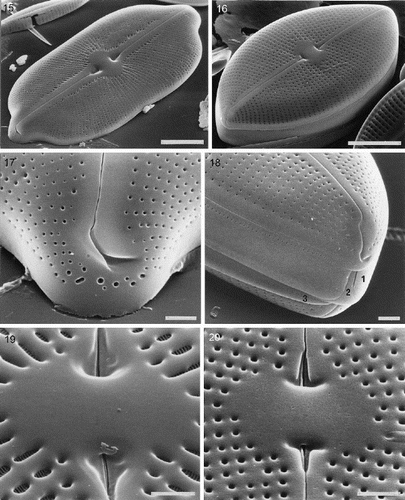
Figs 15–20. External valve structure of Petroneis species, acid cleaned material, SEM. . P. humerosa: exterior of valve. . P. marina: exterior of intact frustule. . P. humerosa: pole and hooked polar raphe ending. . P. marina: pole, showing three girdle bands (1–3) and polar raphe endings in an intact frustule. Note how the terminal raphe fissure descends close to the edge of the valve, interrupting the areola pattern, in contrast to P. humerosa, P. latissima and P. monilifera. . P. monilifera: spathulate groove containing the central raphe endings and central area. Note that the cribra are visible within the large areolae, in contrast to P. humerosa, P. marina and P. latissima. . P. marina: T-shaped central raphe endings and central area. Scale bars represent 10 µm (, ) or 2 µm (–).
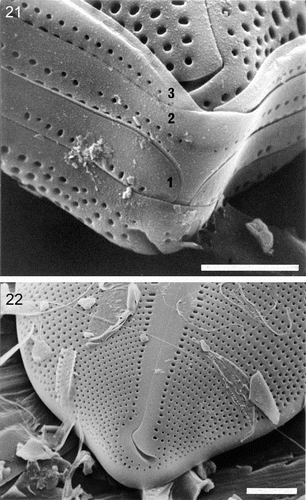
Figs 21–22. External valve structure of Petroneis species, acid cleaned material, SEM. . P. monilifera frustule showing three porous girdle bands (1–3), with open ends arranged alternately. The valvocopula has a single series of poroids, whereas the other two bands have a double row of poroids. . P. latissima: pole and hooked polar raphe ending. Note the similarity to P. humerosa (). Scale bars represent 10 µm () or 5 µm ().
Figs 23–29. Internal valve structure of Petroneis species, acid cleaned material, SEM. . P. humerosa, whole valve interior. . P. marina, whole valve interior. . P. humerosa: internal structure of pole, where the raphe terminates in a helictoglossa. –. Internal views of the central area, showing hooked raphe endings and volate occlusions of the areolae in P. humerosa (), P. latissima (), P. monilifera () and P. marina (). Scale bars represent 10 µm (, ) or 2 µm (–).
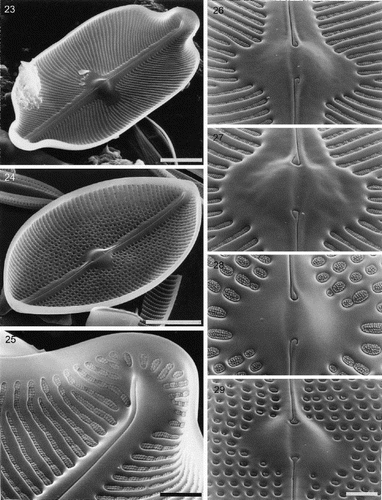
Figs 30–32. Partially developed valves of Petroneis species, acid cleaned material, SEM. , . P. humerosa: deposition of silica is incomplete and cribra have not yet developed. Although the striae are clearly developed, the areolae have not yet been clearly demarcated in all areas by the formation of cross walls within the striae. The central endings of the interior raphe opening are straight, unlike the hooked structure present in fully silicified valves. . P. monilifera: early stages of cribrum development. The striae and areola walls are fully developed and small peg-like ingrowths have formed around the periphery of individual areolae. Scale bars represent 2 µm.
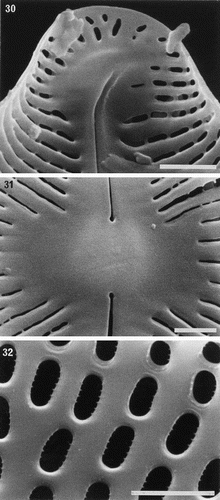
Figs 33–44. Comparison of protoplast structure in interphase cells of Petroneis, Lyrella and Navicula sensu stricto, LM, DIC optics. Adjacent pairs of illustrations show protoplast structure at two focal planes of a single cell, immediately below the upper valve (left), and in the horizontal median plane (right). The lower part of the protoplast is not illustrated, because it resembles that of the upper protoplast in each of these species. , . P. humerosa. Note the thin, transverse bridge (arrowed) that links the four arms of the H-shaped chloroplast; also the pyrenoids (p), nucleus (n) and volutin granules (v). , . P. monilifera. , . P. latissima. Note the thin mucilage sheath that envelopes the cell (arrow), and the numerous small granules, which are frequently observed in this species. , . P. marina. Note the single, square pyrenoid at the centre of the chloroplast, and the rounded nucleus. , . Lyrella species (cf. L. lyra (Ehrenb.) Karayeva). The elaborately lobed chloroplast contains two rounded pyrenoids located beneath the raphe, and a transapically elongate nucleus. , . Navicula reinhardtii Grun. in Van Heurck, with two simple chloroplasts, one lying along each side of the girdle. Scale bars represent 20 µm.
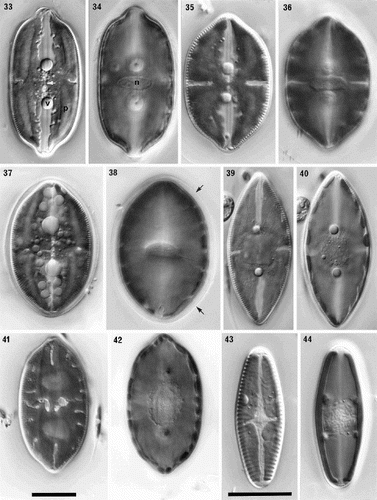
Figs 45–52. Sexual reproduction in Petroneis humerosa from Portobello beach, Edinburgh, LM, DIC optics. . Recently formed auxospores and gametangial thecae within a thick mucilage sheath. The junctions between the fused gametes are still visible (arrow) within each auxospore. . Same cells as in , but two days later. Integration of the gametes is complete and the auxospores are retracting towards each of the gametangial frustules. Note the double boundary of the mucilage sheath (arrows). . Spherical, binucleate auxospores after plasmogamy and retraction to within the gametangial thecae. . Same cells as in . Each auxospore has expanded along the longitudinal axis of the parent theca and developed a silicified perizonium around the cell. The protoplast has pulled inwards, away from the perizonium (arrows). . Expanded auxospore showing a lightly silicified transverse perizonium in section at left, and the central longitudinal band (striations right of centre). . Near-surface focus of expanded auxospore, showing transverse perizonium. Note the straight, central band (white arrows) and the other curved bands, which appear to be interrupted at the longitudinal suture (between black arrows). . Different focus of the cell shown in . Two H-shaped chloroplasts lie around the periphery on opposite sides of the auxospore. The nuclei (still unfused) lie beneath the intersection of the longitudinal suture and the central transverse band of the perizonium. . Expanded auxospore of Navicula oblonga. Note the caps of organic material at each pole, and the linear-concave outline of the auxospore. Scale bars represent 20 µm.
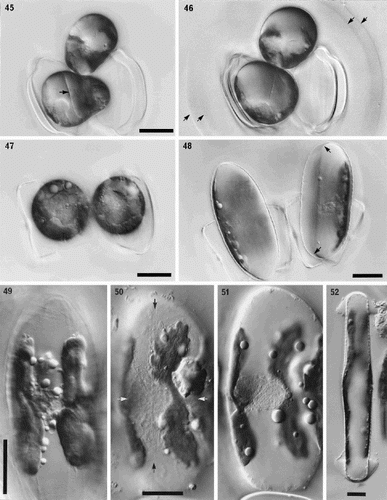
Fig. 53. Phylogram of selected raphid diatoms (with Rhizosolenia, Thalassiosira and Eunotia as outgroups) inferred from maximum likelihood (ML) analysis of rbcL sequences. Numbers on branches are (in order) bootstrap values from maximum parsimony analysis, bootstrap values from ML analysis, and posterior probabilities from Bayesian analysis, all expressed as percentages.
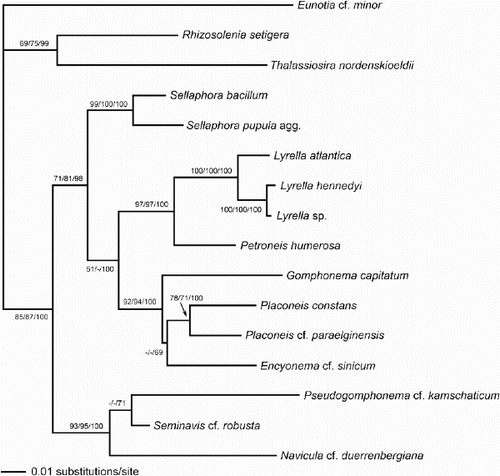
Fig. 54. Majority rule consensus tree (length 70, consistency index 0.49, retention index 0.64) based on a maximum parsimony analysis of the morphological, cytological and reproductive data matrix in ; 11 most parsimonious trees were found. Numbers above branches indicate the percentage frequency of occurrence of the node among the most parsimonious trees. Italicised numbers below branches are bootstrap values.
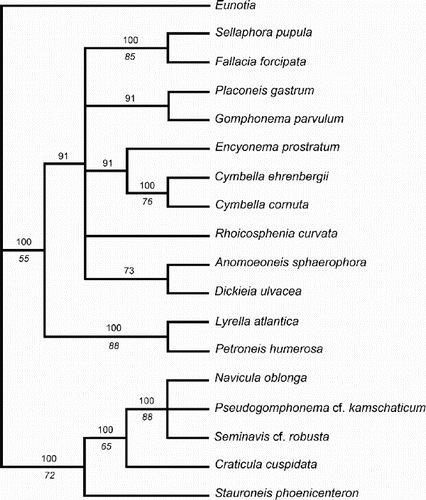
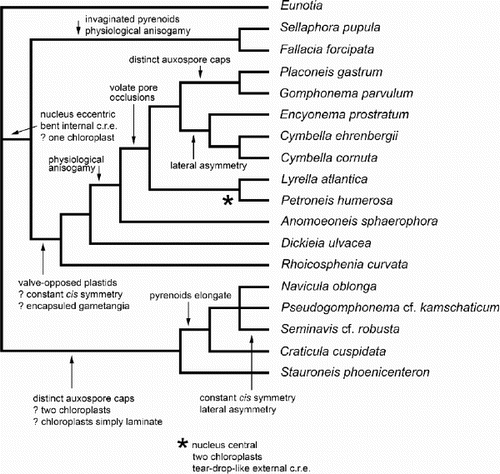
Fig. 55. One of the most-parsimonious trees derived from analysis of the matrix. The tree topology is similar to the molecular phylogram () and is used to illustrate some possible character state transitions, following character optimisation using MacClade version 3.07. Queries indicate that the character state transition cannot be placed unequivocally, but cannot be any higher in the tree without incurring a parsimony penalty. c.r.e = central raphe endings.