Figures & data
Table 1. Collection sites and dates at which species of the diatom genera Haslea, Gyrosigma and Pleurosigma were isolated for molecular analyses. GenBank Accession numbers are indicated
Figs 1 – 5. Light and scanning electron micrographs of Haslea nipkowii from the French Atlantic coast. Figs 1, 2. Cells with two plate-like chloroplasts along margins. Fig. 3. Living cell in girdle view. Fig. 4. Cleaned valve in phase contrast optics. Fig. 5. External valve view in SEM. All scale bars represent 10 μm.
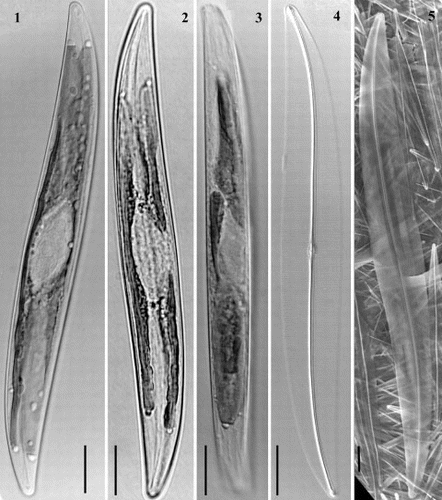
Figs 6 – 8. Light micrographs of Haslea nipkowii from the French Atlantic coast. Fig. 6. Half valve showing a distinct white spot at centre (arrowhead) with opposing thin central bar (arrow) and wide axial costa. Fig. 7. Central valve showing transapical striae crossed at right angle by a longitudinal pattern with distinct white spot (arrowhead) and opposing narrow central bar (arrow). Fig. 8. Apex showing bifurcated distal raphe fissure. All scale bars represent 10 μm.
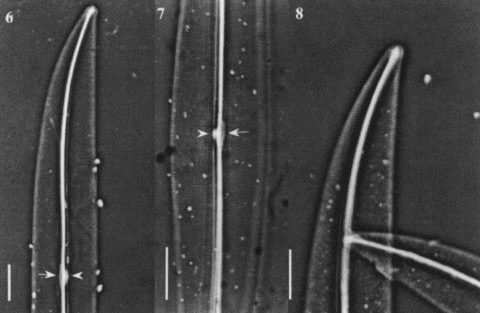
Figs 9 – 12. Light micrographs of Gyrosigma spp. Figs 9, 10. Gyrosigma nipkowii (holotype slide # 3203024 from Meister collection (Z), Bay of Amoy, China). Fig. 9. Whole valve showing a distinct white spot at centre (arrowhead) with opposing central bar (arrow) and wide axial costa. Fig. 10. Broken valve. Figs 11, 12. Gyrosigma pallidum (holotype slide G.C. # 62630 from Riznyk collection (ANSP), Yaquina Estuary, USA). Whole valve exhibiting a distinct axial costa, a narrow central bar (arrow) with an opposing white spot (arrowhead). All scale bars represent 10 μm.
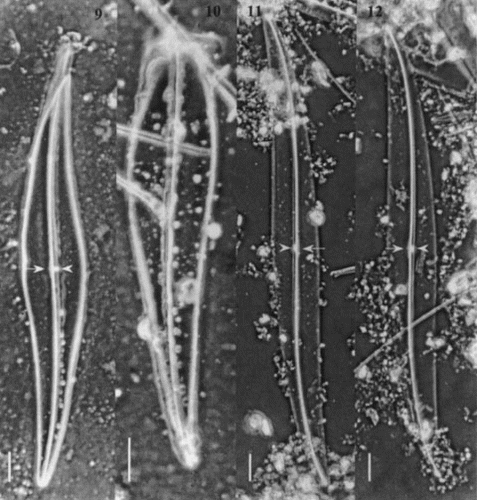
Figs 13 – 19. Light micrographs of Gyrosigma spp. Figs 13 – 15. Gyrosigma nipkowii (holotype slide # 3203024). Fig. 13. Half valve. Fig. 14. Valve centre showing axial costa, narrow central bar with opposing white spot. Note the slightly deflected proximal raphe ends toward the primary side. Fig. 15. Valve apex. Figs 16 – 19. Gyrosigma pallidum (holotype slide G.C. # 62630). Fig. 16. Half valve. Figs 17, 18. Centre of valve exhibiting a distinct axial costa, a narrow central bar (arrow) with an opposing white spot (arrowhead). Note striae pattern crossed at 90°. Fig. 19. Valve apex showing T-shaped distal raphe fissure (arrow). Scale bars represent: Figs 13 – 18, 10 μm; Fig. 19, 5 μm.
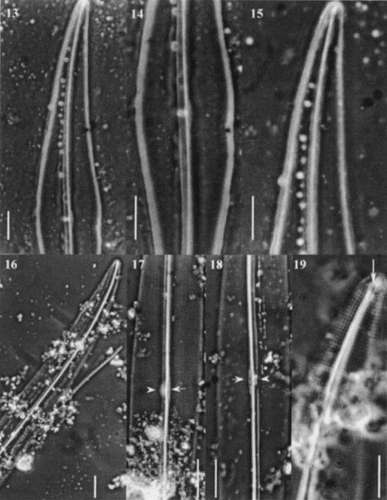
Figs 20 – 28. Scanning electron micrographs of Haslea nipkowii from the French Atlantic coast. Fig. 20. Internal valve view midway between apex and centre showing the stria pattern and the axial costa completely overhanging the raphe system. Fig. 21. Internal valve at centre showing the proximal raphe ends, the narrow central bar (black arrowhead) with opposing bulge (white arrowhead) which correspond to the white spot visible in LM. Fig. 22. Valve centre in internal view exhibiting small transapical extensions of the central bar. Figs 23, 24. Internal views of apex showing the quadrangular areolae, the straight helictoglossa and the two peripheral longitudinal slits reuniting at the far tip of the valve. Note the recessed axial costa in Fig. 24. Fig. 25. Internal valve centre showing roundish areolae, a recessed axial costa, straight proximal raphe ends and a narrow central bar. Fig. 26. External view of valve at centre showing the longitudinal slits, the wider primary side and the proximal raphe ends slightly deflected toward the same side and gently overlapping. Fig. 27. Internal view of valve showing the axial costa and the first series of areolae with the foramen opening at an angle (arrow). Fig. 28. Same specimen tilted (46°) showing the foramen of the first series of areolae (arrow).
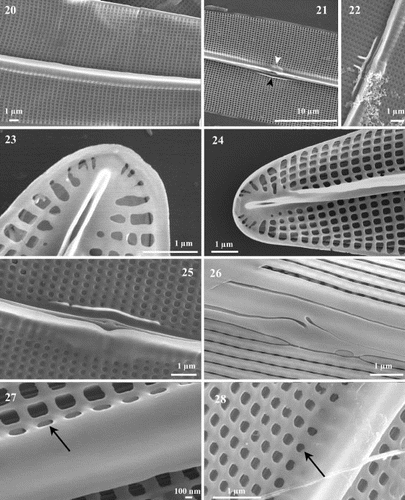
Figs 29 – 32. Scanning electron micrographs of Haslea nipkowii from the French Atlantic coast. Fig. 29. External valve view midway between apex and centre showing the wider axial area on the primary side bordered by the first longitudinal slit which appears unopened because of the angled position of the foramen underneath. Figs 30, 31. Apex in external view showing the uneven T-shaped terminal fissure of the raphe, the terminal area in the shape of a Bishop's mitre, the abutting longitudinal slits with the two peripheral slits that merge together at the far tip of the pole. Fig. 32. Internal view of apex showing the straight helictoglossa and the merging of the two peripheral slits.
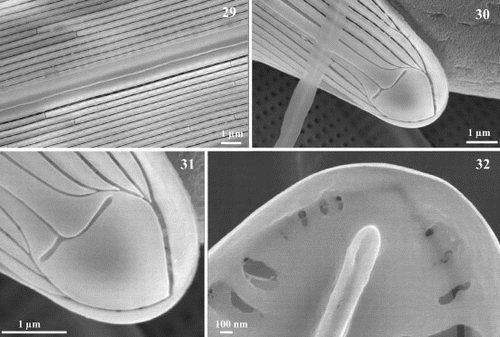
Figs 33 – 40. Scanning electron micrographs of transapical thin sections of Haslea nipkowii. Fig. 33. Thin section midway between apex and centre showing the general structure of the frustule. Fig. 34. Thin section near the apex. Figs 35 – 37. Thin sections midway between apex and centre. Details showing the larger areola with internal and external openings (single arrow), the C-cedilla (Ç) shape of the raphe fissure (single line) and the prominent and overhanging axial costa (double arrow). Fig. 38. Thin section at valve centre showing the large areola, the simpler raphe fissure (single line), the recessed axial costa (double arrow) and the slightly raised central bar (double line). Figs 39, 40. Thin sections showing the cingular valvocopula and copula. Scale bars represent: Figs 33 – 35, 37, 38, 1 μm; Figs 36, 39, 40, 100 nm.
Fig. 41. I – III: Numbering scheme (I) and structures of C25 HBI alkenes characterized for Haslea nipkowii. I – II: Structures of C25 HBI alkenes previously characterized for Haslea species. IV – V: Structures of C25 HBI alkenes previously characterized for sigmoid taxa.
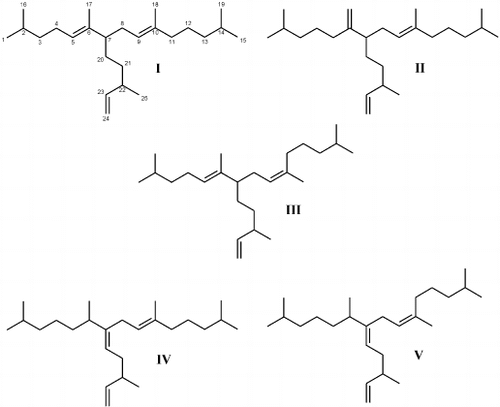
Fig. 42. Partial total ion current chromatogram of the non-saponifiable lipid fraction of the diatom Haslea nipkowii showing the presence of three C25 HBI isomers, n-C21:6 (heneicosahexaene) and phytol, the side chain of chlorophyll.
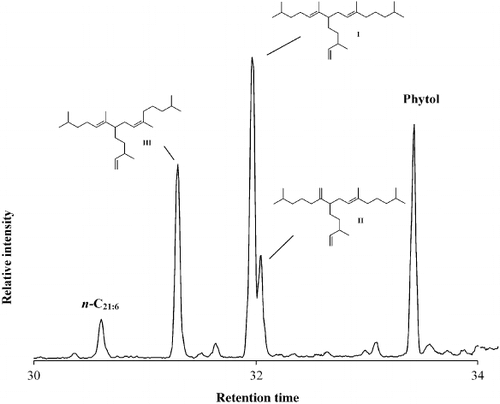
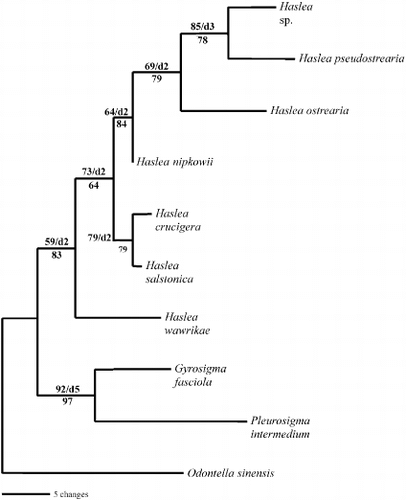
Fig. 43. Single most parsimonious tree for 16S sequences of Haslea, Gyrosigma and Pleurosigma species, rooted with Odontella sinensis. The tree is 104 steps long with a consistency index of 0.7885 and a retention index of 0.6000. Branch lengths are proportional to the nucleotide changes indicated by the scale bar below the tree. Numbers above or below branches are bootstrap values based on 1000 replications of the MP or ML analyses, respectively. Decay index values (d) are also provided.