Figures & data
Fig. 1. Variables used for the conventional morphometric analyses, illustrated on a C. meneghiniana valve. D1 – valve diameter; D2 – valve diameter measured between the inner edges of the mantle (i.e., D1 minus twice the mantle thickness); D3 – diameter of the central area; RW – width of the rimoportula; RL – length of the rimoportula; NC – number of costae; MFP – number of marginal fultoportulae; CFP – number of central fultoportulae.
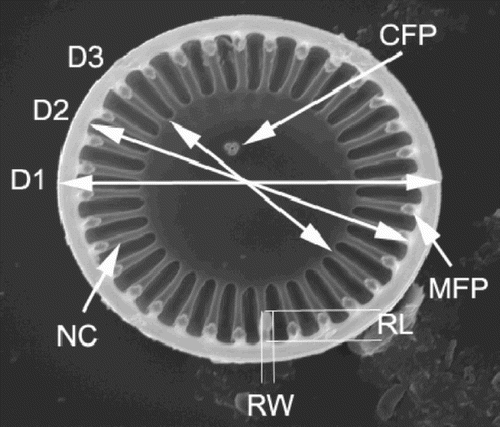
Fig. 2. Positions of landmarks 2 to 9 used for the geometric morphometric analyses shown on a valve from the ‘ambiguous’ morph. Landmark 1, the midpoint of the valve is not shown here. describes positioning of the landmarks.
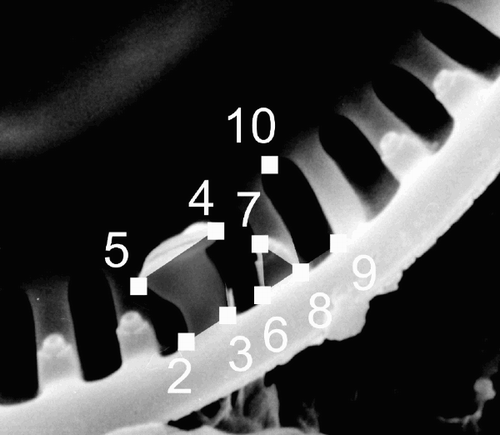
Table 1 . Landmark points recorded for the geometric morphometric analyses. Their position is described on a valve, the RP of which was oriented towards the bottom of the image. See also
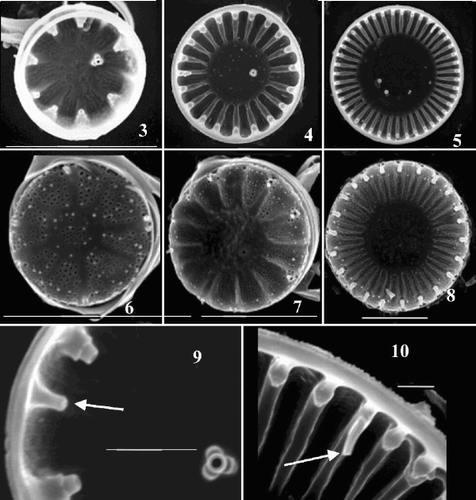
Figs 3–10. Scanning electron micrographs of valves from Cyclotella meneghiniana cultures. –. Valve interiors. –. Valve exteriors over the size range. –, Detail of interior margins showing rimoportulae (arrows) in an extremely small () and a larger () valve. Scale bars represent 5 µm (–) and 1 µm (, ).
Figs 11–18. SEM images of valves of the ‘ambiguous’ morph of Cyclotella scaldensis. –. Valve interiors. –. Valve exteriors over the size range. . Interior margin showing rimoportula. . Exterior margin showing process openings; RP opening is marked by an arrow. Scale bars represent 5 µm (–) and 1 µm (, ).
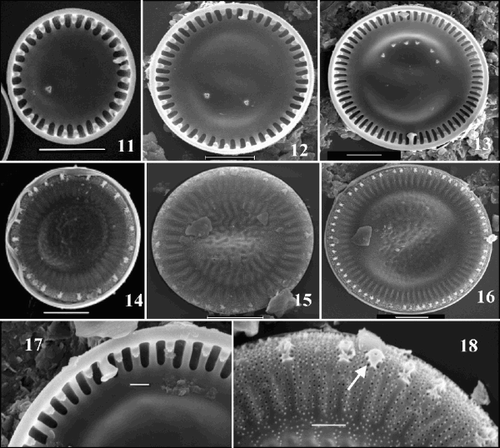
Figs 19–26. SEM images of valves of the ‘extreme’ morph of Cyclotella scaldensis. –. Valve interiors. –. Valve exteriors over the size range. . Exterior margin, showing process openings; RP opening marked by an arrow. . Interior margin with RP and two neighbouring FPs. Scale bars represent 5 µm (–) and 2 µm (, ).
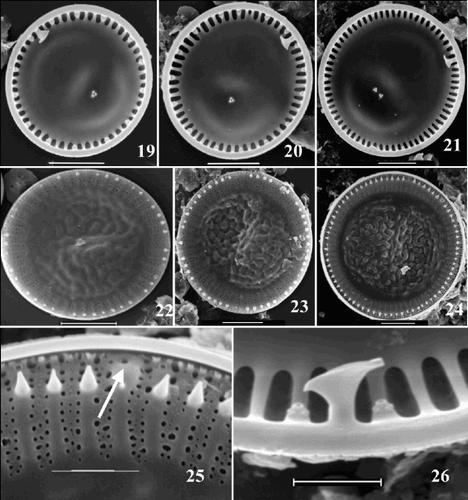
Fig. 27. Scatter plot of number of costae (NC) against number of marginal fultoportulae (MFP). Group outliers are connected by lines. (+ = C. meneghiniana, = ‘ambiguous’ morph of C. scaldensis, ○ = ‘extreme’ morph of C. scaldensis. c = cultures, g = field samples from the River Geeste, s = field samples from the river Schelde.)
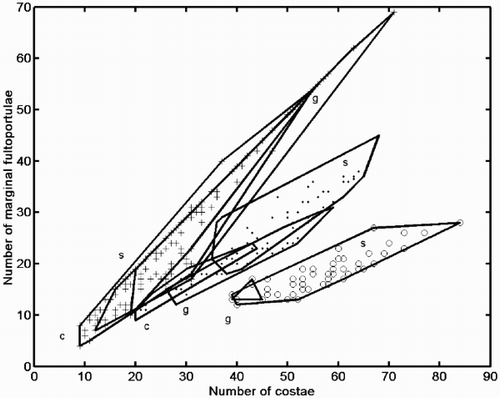
Fig. 28. Scatter plot on the first two principal component axes of the conventional morphometric dataset. Group outliers are connected by lines. (+ = C. meneghiniana, = ‘ambiguous’, ○ = ‘extreme’ morph of C. scaldensis. c = cultures, g = field samples from the River Geeste, s = field samples from the river Schelde.) Vector correlations of the original variables with the first two principal components are shown in the upper left hand corner.
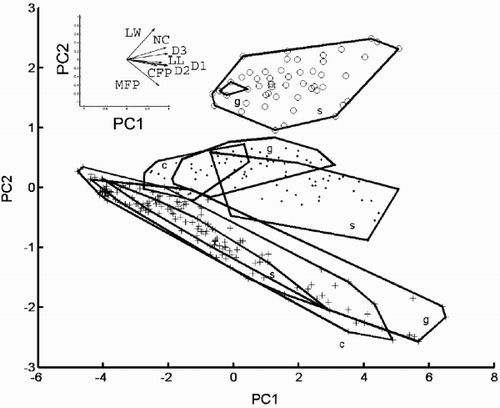
Fig. 29. Reconstructed morphologies of some points in the plane of the first two principal component axes (data shown in ). Group outliers of the three morphs are shown. Morphologies were ‘reconstructed’ by projecting PCA scores back into the coordinate system of the original variables and drawing diagrams based on the resulting values (see ‘Materials and methods’ for details). The diagrams are drawn at the same scale. The morphologies shown correspond to PCA scores (−4, 0), (0, −1), (0, 0.5), (0, 1.5), (2, 0), (2, 2), (4, 0), (4, 2) and (6, −2).
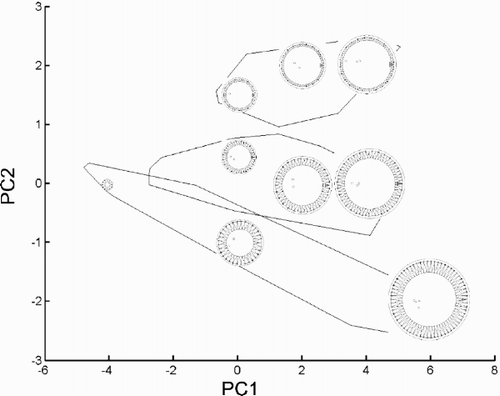
Fig. 30. Canonical variate analysis of the conventional morphometric dataset. Canonical variate scores calculated by grouping the specimens according to morph. (+ = C. meneghiniana, = ‘ambiguous’, ○ = ‘extreme’ morph of C. scaldensis; c = cultures, g = field samples from the River Geeste, s = field samples from the river Schelde.) Group outliers are connected by lines. Vector correlations of the canonical variates with the eight original variables are shown in the upper right hand corner.
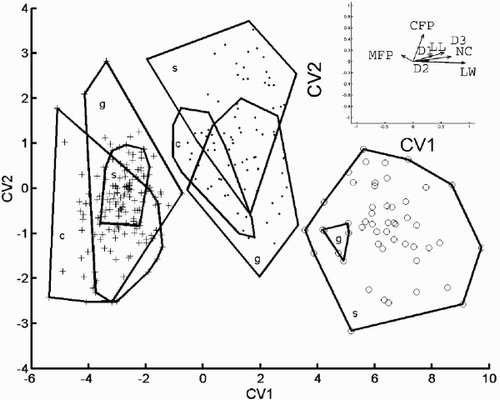
Fig. 31. Relative warp analysis with α = −1, small scale differences weighted more than global ones. Scores of specimens on the first two relative warp axes. Group outliers are connected by lines. (+ = C. meneghiniana, = ‘ambiguous’, ○ = ‘extreme’ morph of C. scaldensis; c = cultures, g = field samples from the River Geeste, s = field samples from the river Schelde.)
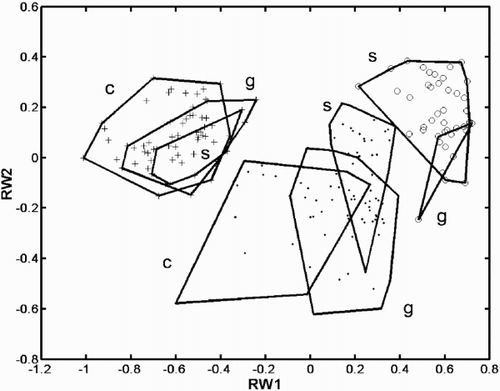
Fig. 32. Landmark configurations corresponding to some points of depicted as deformations from the mean configuration (the origin of the coordinate system in ). corresponds to the point (0, 0.5) of the coordinate system, to (−0.5, 0), to (0.5, 0), and to (0, −0.5). The points connected by thick lines represent the landmark points shown in , but the diagrams are rotated approximately 90° anti-clockwise compared to .
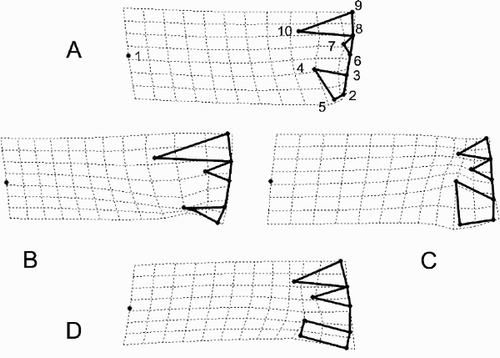
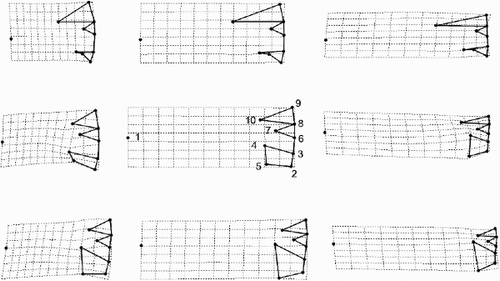
Fig. 33. Patterns of allometric valve shape change in the three morphs with size as calculated from a linear regression of the partial warp scores on the logarithm of valve diameter. Landmarks (see to link them to the anatomic features of the valve interior) are connected by thick lines but the diagrams are rotated approximately 90° anti-clockwise compared to . The landmark configurations predicted by the regression for three different diameters (D1 = 8, 16 and 24 µm, respectively) in the three morphs are shown in the columns and the different taxa by rows. Row 1 = Cyclotella meneghiniana, row 2 = ‘ambiguous’ morph of C. scaldensis and row 3 = ‘extreme’ morph of Cyclotella scaldensis.