Figures & data
Table 1. Clones of Ditylum brightwellii used in two mating system experiments. The mean diameter of each clone at the beginning of each experiment (n = 100 cells per clone) is given. Two clones, N3 and N4, were used in both experiments, and denoted as N3′ and N4′ in the second experiment.
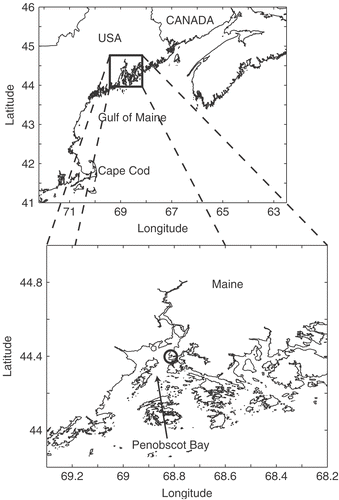
Fig. 1. Sampling location within the Gulf of Maine. The inset shows Penobscot Bay and Wadsworth Cove (circle).
Figs. 2–10. Life-cycle stages in Ditylum brightwellii. . Live egg (arrow) and sperm (arrowhead) and auxospore (double arrow). . Fixed egg viewed with bright-field microscopy. . The same egg stained with DAPI. . Mass spermatogenesis in a live culture. Note swelling of spermatogonia (arrow). . Spermatogonangium with rudimentary thecae (arrow) in 1% glutaraldehyde and formaldehyde fixative. . Early stage auxospores in living preparation. Note properizonium (arrows). . Auxospores developed fully; formation of both valves of the initial cells is nearly complete (living preparation). . Resting spore of Ditylum brightwellii in a clonal culture from a natural population at Wadsworth Cove (fixed sample). . Vegetative cell enlargement in living Ditylum brightwellii. Scale bars: 10 µm.
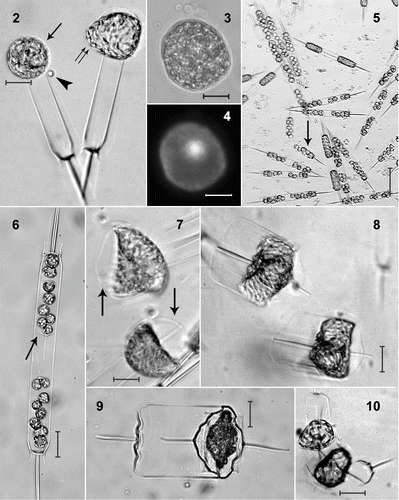
Fig. 11. Life history of Ditylum brightwellii. Size reduction occurs over time via vegetative cell division. Resting spores develop from, and germinate into vegetative cells (Hargraves, Citation1984). Sexual reproduction takes place between haploid eggs and sperm; the auxospore (diploid) is a specialized zygotic cell.
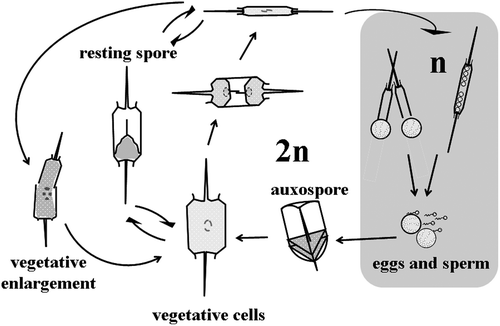
Fig. 12. Natural logarithm of the ratio of production of oogonia to spermatogonangia (O:S) in Ditylum brightwellii. Each clone is represented by its diameter measured on day 0. The ratio is the average of the duplicated treatments over the entire experiment ± SD (n = 16). The clones with the open symbol (○) did not produce gametes.
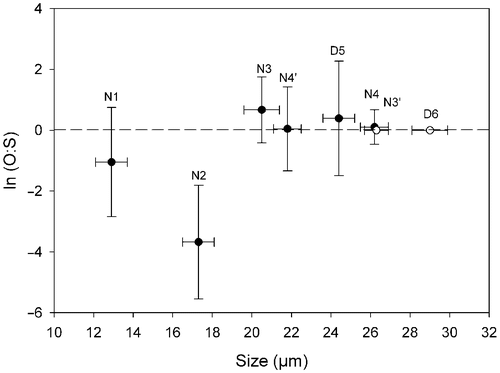
Fig. 13. The frequency (%) of gamete production in three mating crosses of Ditylum brightwellii over 7 days. Shaded areas represent the area under the curve of the mean of duplicate measurements (filled circles). Trends in gamete production are highlighted with the upper solid line representing the mean multiplied by five.
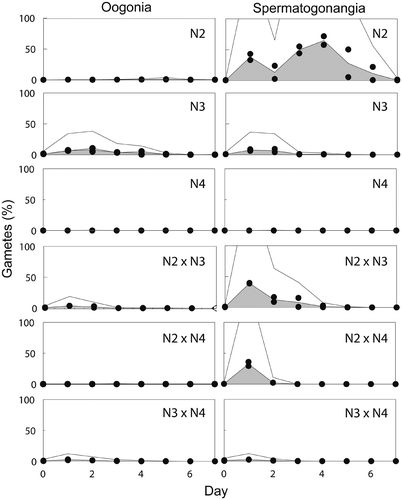
Fig. 14. Frequency (%) of auxospores produced in three mating crosses of Ditylum brightwellii over 7 days. Shaded areas represent the area under the curve of the mean of duplicate measurements (filled circles). Trends in gamete production are highlighted with the upper solid line representing the mean multiplied by five.
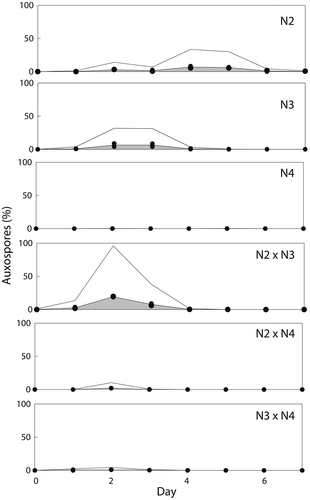
Fig. 15. Abundance of Ditylum brightwellii at Wadsworth Cove, autumn 2004. Mean number of cells l−1 ± SD (○ indicates days on which spermatogonangia were absent).
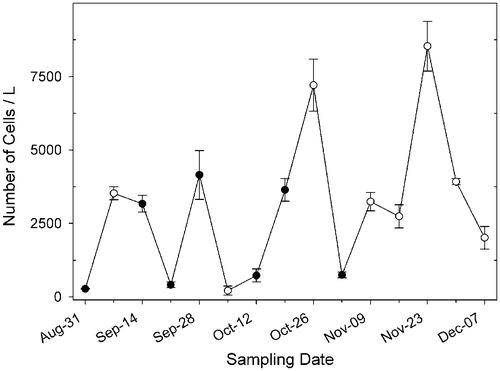
Fig. 16. Size frequency distributions of populations of Ditylum brightwellii from Wadsworth Cove by sampling date. The stars denote presence of spermatogonangia and the dotted line is the upper size limit for gametogenesis observed in lab experiments.
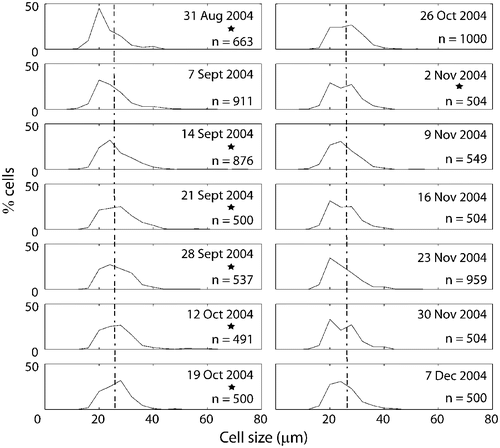
Fig. 17. Changes in mean, median and principal mode of Ditylum brightwellii from the Wadsworth Cove population during autumn 2004.
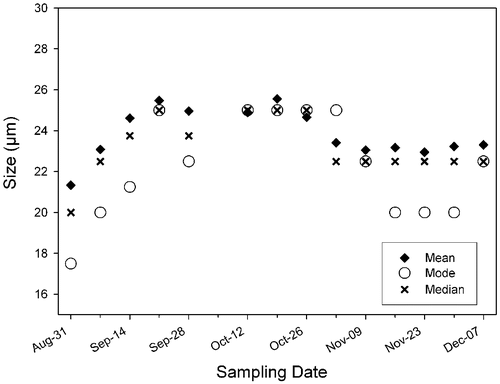
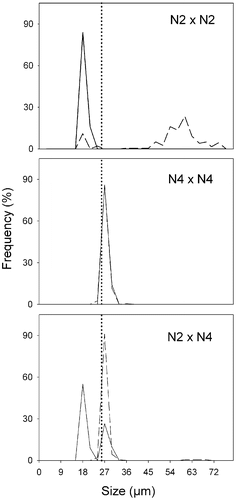
Fig. 18. Size distributions in Ditylum brightwellii N2 × N4 cross over time. Straight line represents day 0, dashed line represents day 5. Day 0, n = 50 (intraclonal crosses), n = 200 (interclonal crosses); day 5, n = 200 (all treatments). The dotted line is the upper size limit for gametogenesis observed in lab experiments.