Figures & data
Figs 1–18. Fluorescence microscopic observation of various mitochondria during zygote maturation in the dark. . Fluorescence micrographs of zygotes stained with DiOC6 (), SYBR Green I (), and both SYBR Green I and DiOC6 simultaneously (). . Images focused on the surface of a single zygote. . Images focused on the inside of a single zygote. The images of the cells were taken 0 (), 3 (), and 7 days () after transfer to darkness. Dark treatment for zygote maturation started immediately after day 1 of zygote formation. Asterisk: cell nucleus. Arrow: chloroplast nucleoid. Arrowhead: mitochondrial nucleoid. Scale bar: 5 µm.
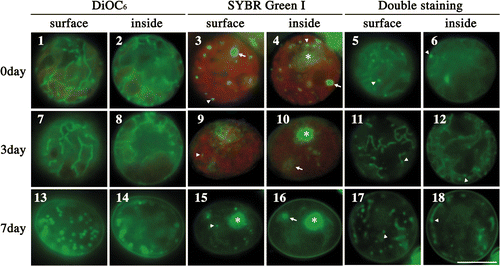
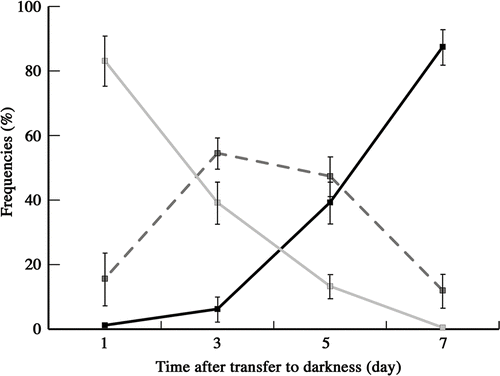
Fig. 19. Frequencies of zygotes classified into three types according to mitochondrial morphology after transferring young zygotes to darkness: zygotes with mesh-like mitochondria (grey line), zygotes with fragmented mitochondria (dashed line), and zygotes with particle-like mitochondria (black line). Mitochondrial features were determined by fluorescence microscopy. Standard deviations were calculated from three independent experiments. 311, 245, 220, 332 cells were counted at 1, 3, 5, 7 days, respectively.
Figs 20–61. Fluorescence micrographs of mature zygotes after light exposure. Fluorescence micrographs of zygotes stained with DiOC6 (), SYBR Green I (), and both SYBR Green I and DiOC6 simultaneously (). . Images focused on the surface layer of a single zygote. . Images focused on the inside of a single zygote. The images were taken approximately 0 h (), 3 h (), 7 h (), 11 h (), 14 h (), 16 h () and 20 h () after light exposure. Asterisk: cell nucleus. Arrow: organellar nucleoid. Arrowhead: mitochondrial nucleoid. Scale bar: 5 µm.
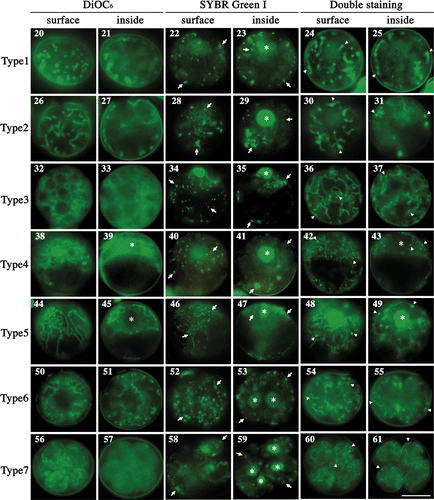
Fig. 62. Frequencies of mature zygotes classified into seven types according to mitochondrial morphology after light exposure. Mitochondrial morphology was determined by fluorescence microscopy. Standard deviations were calculated from three independent observations. 332, 295, 291, 586, 666, 471, 171, 221 cells were counted at 0, 3, 6, 9, 12, 15, 18, 21 h, respectively.
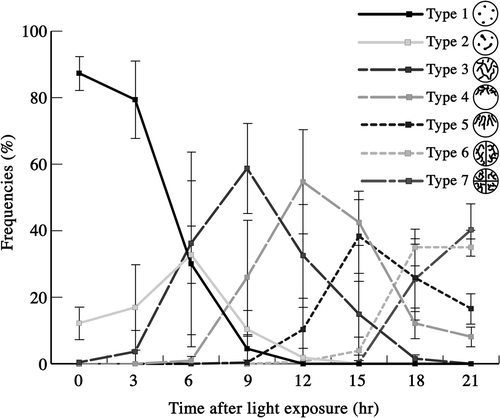
Figs 63–71. Changes in nucleoid morphology after exposing mature zygotes to light. Fluorescence micrographs of nucleoids in mature zygotes after light exposure. Zygotes were stained with SYBR Green I. . Fluorescence micrographs of a zygote. . An enlarged portion of a nucleoid shown by an arrow in , respectively. Images taken approximately 0 h (), 8 h () and 10 h () after light exposure. Arrow: particle-like or stringy nucleoid. Arrowhead: newly produced nucleoid. Scale bars: 5 µm (); 1 µm (). . Frequencies of mature zygotes classified into three types according to the morphology of the nucleoid after light exposure: zygotes with particle-like nucleoids with no apparent production of new nucleoids (see ) (dashed line), zygotes with less than five stringy nucleoids with apparent low production (see ) (black line), and zygotes with more than five stringy nucleoids or long stringy nucleoids with apparent high production (see ) (grey line). Standard deviations were calculated from three independent experiments. 108, 70, 247, 144, 145, 228, 155, 303, 256, 161, 126 cells were counted at 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 h, respectively.
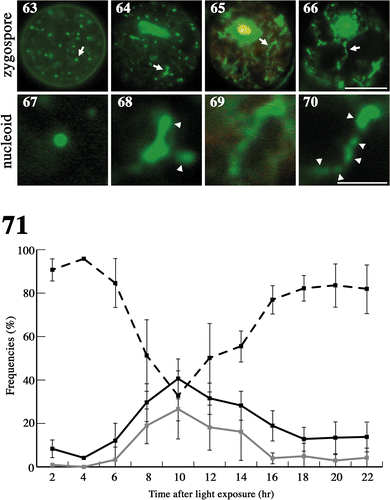
Fig. 72. Measurement of zygote oxygen consumption. Oxygen consumption was measured from the beginning of zygote maturation until the completion of meiosis. Black arrow: zygotes were transferred to the dark for maturation. Grey arrow: mature zygotes were exposed to light to induce meiosis. Standard deviations were calculated from five independent experiments.
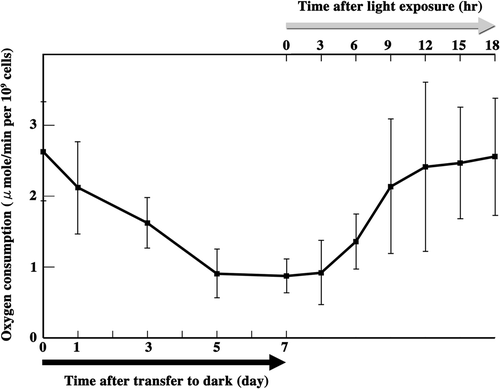
Figs 73–87. The effects of demecolcine on mitochondria during meiosis. . The frequency of each type of mitochondria was determined by fluorescence microscopy. Zygotes treated with demecolcine at concentrations of 2.5 µg/ml () and 25 µg/ml () after light exposure. Standard deviations were calculated from three independent experiments. 96, 174, 210, 244, 194, 100 cells were counted at 3, 6, 9, 12, 15, 18 h, respectively (). 69, 168, 326, 212, 198, 139 cells were counted at 3, 6, 9, 12, 15, 18 h, respectively (). . Fluorescence micrographs of zygotes treated with demecolcine. Cells were stained with DiOC6. . Images focused on the surface layer of a single zygote. . Images focused on the inside of a single zygote. The concentration of demecolcine was 2.5 µg/ml () or 25 µg/ml (). Micrographs were taken approximately 9 h (), 12 h (), 15 h (), and 18 h () after light exposure. Scale bars: 5 µm. . The effect of demecolcine on the oxygen consumption of mature zygotes after light exposure. Black solid line, 25 µg/ml demecolcine; black dashed line, 2.5 µg/ml demecolcine; grey solid line, no treatment (). Standard deviations were calculated from five independent experiments.
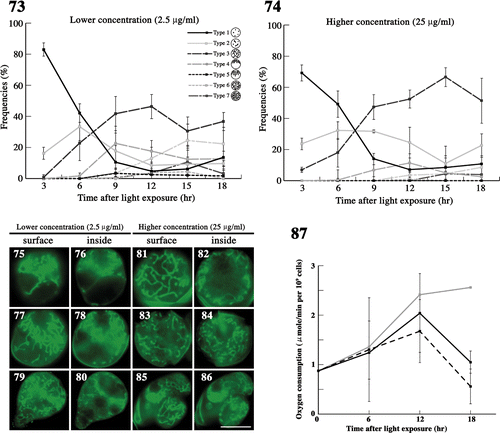
Figs 88–102. The effects of latrunculin B on mitochondria during meiosis. . The frequency of each type of mitochondria was determined by fluorescence microscopy. Zygotes treated with latrunculin B at 30 nM () and 300 nM () after light exposure. Standard deviations were calculated from three independent experiments: 74, 169, 122, 117, 283, 129 cells were counted at 3, 6, 9, 12, 15, 18 h, respectively (); 89, 270, 154, 190, 226, 85 cells were counted at 3, 6, 9, 12, 15, 18 h, respectively (). . Fluorescence micrographs of zygotes showing mitochondria treated with latrunculin B. Cells stained with DiOC6. . Micrographs of the surface layer of a single zygote. . Micrographs of the inside of a single zygote. The concentration of latrunculin B was 30 nM () or 300 nM (). The micrographs were taken approximately 6 h (), 15 h (), and 18 h () after light exposure. Arrow: aggregation of mitochondria. Arrowhead: loop-like mitochondria. Scale bars: 5 µm. . The effect of latrunculin B on the oxygen consumption of mature zygotes after light exposure. Black solid line, 300 nM latrunculin B; black dashed line, 30 nM latrunculin B; grey solid line, no treatment (). Standard deviations were calculated from five independent experiments.
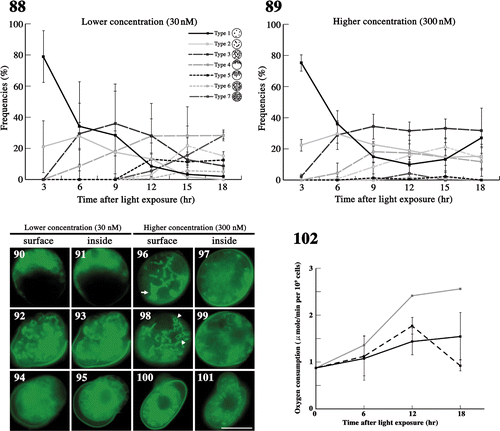
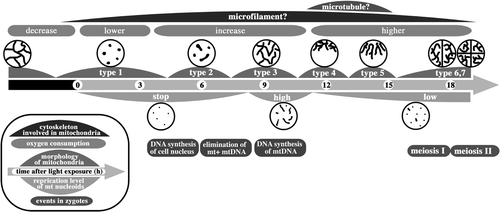
Fig. 103. Dynamics of mitochondria and their nucleoids during pre-meiosis and meiosis in zygotes. Particle-like mitochondria (type 1) that are fragmented during maturation connect to form tubular structures (type 2) after light exposure. The tubular networks in whole zygotes (type 3) assemble around the cell nucleus (type 4) and form a radial structure (type 5). These dynamics are controlled by microtubules. Mitochondria spread into two and four progeny cells (type 6 and 7). The high-level replication of mitochondrial nucleoids is synchronized with the formation of the mitochondrial tubular network. Low-level replication continues until tetrad formation.