Figures & data
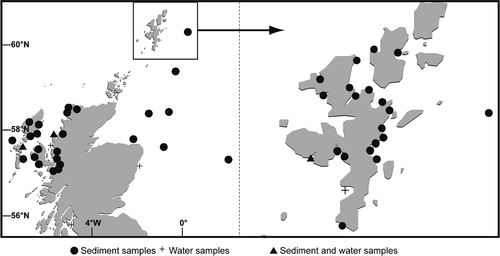
Fig. 1. Distribution of Alexandrium cysts (square root transformed) collected from 1992 to 1998. Cyst data obtained from Lewis et al. (Citation1995) are included. Symbol areas linearly scaled to cyst concentrations.
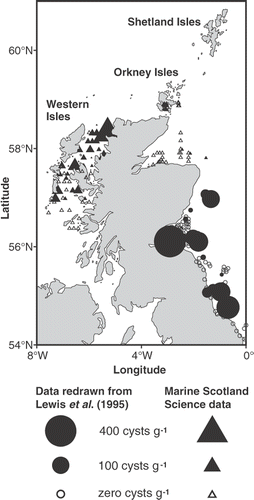
Fig. 2. Maximum annual averaged Alexandrium cell densities (square root transformed) observed from 1996 to 2005 around the Scottish coast. Symbol areas linearly scaled to cell concentrations.
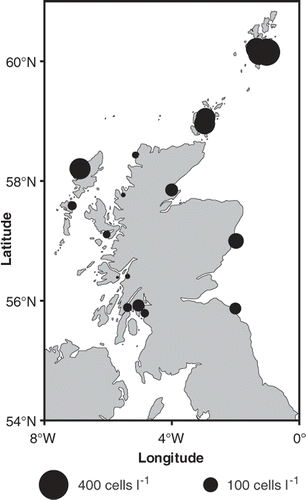
Table 1. Details of Alexandrium cultures from Scotland generated as part of this study.
Fig. 4. Locations of toxin- and non toxin-producing Alexandrium tamarense established in culture during this study. Cultures of A. tamarense Groups I and III detailed in Collins et al. (Citation2009) are included.
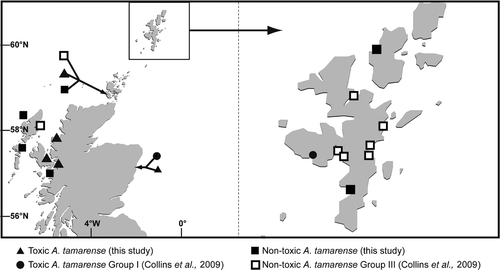
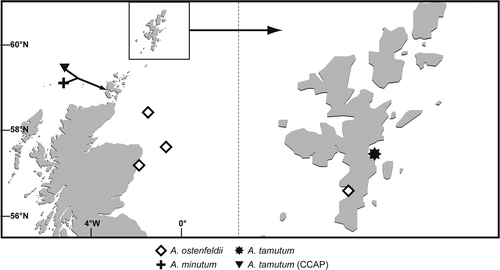
Fig. 5. Locations of A. minutum, A. ostenfeldii and A. tamutum established in culture during this study. Locations of A. tamutum (previously unidentified isolates from CCAP) are included.
Figs 6–15. Alexandrium tamarense from Scottish waters, identified using light microscopy with epifluorescence. . General cell shape in dorsal view showing well developed cingulum. . Ventral-apical view showing attachment of APC with 1′, small ventral pore (Vp) on 1′ and 6′′. . Detailed view of apical pore (Po). . Ventral view showing 1′ and 6′′ and sulcal plates. , . Detailed view of 1′ with small Vp and 6. . Antapical view of Sp with no posterior attachment pore (Pap). . Antapical view showing Sp Pap. Scale bars represent: Figs , , 10 µm; , 5 µm.
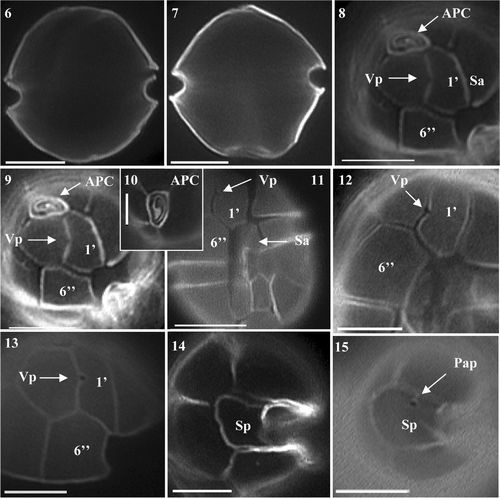
Fig. 16. MRM chromatograms (with variable intensity) from LC-MS/MS analysis of extracts of Alexandrium tamarense isolated from Scottish coastal waters.
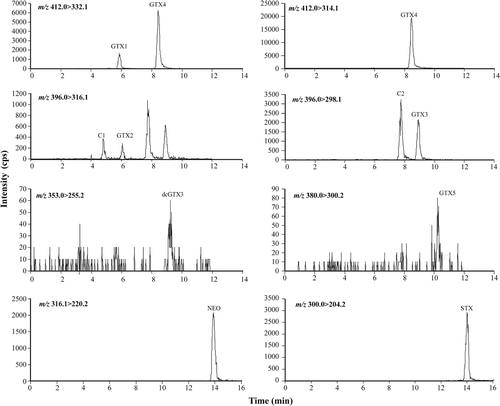
Fig. 17. Histogram detailing percentage contribution of PSP toxin analogues detected in Scottish A. tamarense isolates by LC-MS/MS.
Table 2. Toxins produced by Alexandrium maintained in batch culture in L1 media.
Figs 18–26. Alexandrium ostenfeldii from Scottish waters, identified using light microscopy with epifluorescence. . General cell shape. . Epithecal plates showing clear contact between APC and 1′, with large ventral pore and wide 6′′. . Apical view of APC. . Ventral view showing 1′ with large ventral pore and wide 6′′. , . 1′ with large ventral pore, Sa plate and partial view of 6′′. . Epithecal plates showing APC 1′ and wide 6′′. , . Antapical view showing Sp with no Pap. Scale bars represent: 10 µm.
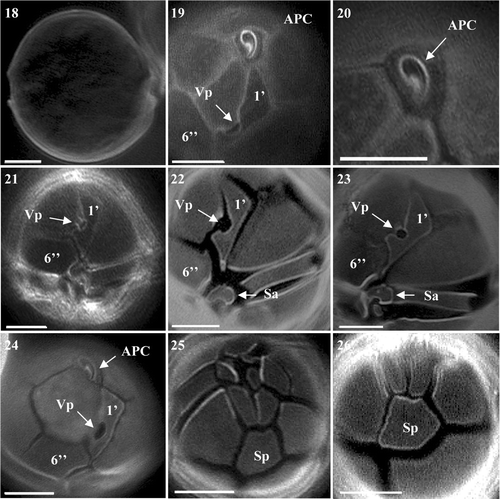
Fig. 28. MRM chromatograms from LC-MS/MS analysis of an extract of Alexandrium ostenfeldii (S06/013/01) isolated from Scottish coastal waters showing the presence of 20-methyl spirolide G.
Fig. 29. Enhanced MS Scan (EMS) in positive mode of peak at 10.6 min (see ) characteristic of 20-methyl spirolide G from an Alexandrium ostenfeldii extract (S06/013/01) isolated from Scottish coastal waters.
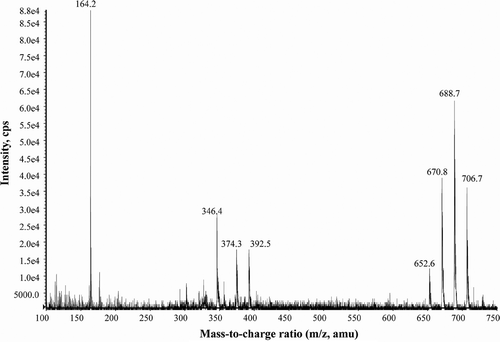
Figs 30–38. Alexandrium minutum from Scottish waters, identified using light microscopy with epifluorescence. . General cell shape. . Dorsal view of cell. . Ventral view showing 1′, 6′′ and Sa. . Ventral view of cell showing sulcal plates and descending cingulum. . Ventral view showing descending cingulum. . Long and narrow 1′ with clear ventral pore and 6′′ plate. , . Narrow 1′ and 6′′ plates. . Antapical view showing Sp with attachment pore. Scale bars represent: Figs , , 10 µm; , 5 µm.
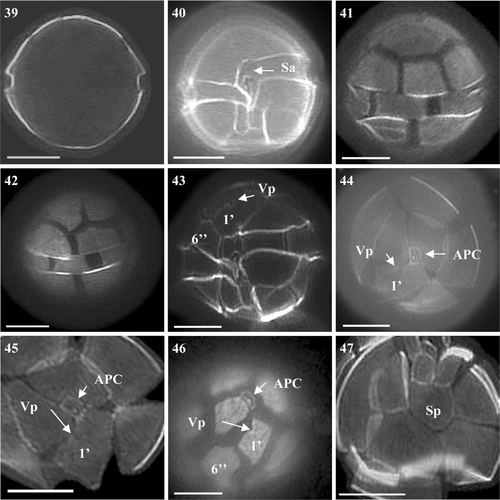
Figs 39–47. Alexandrium tamutum from Scottish waters, identified using light microscopy with epifluorescence. . General cell shape. . Ventral view showing sulcal plates and descending shallow cingulum. , . Dorsal view of cell. . Ventral view showing 1′ with ventral pore and wide 6′′. , . Epithecal plates showing contact between the APC and 1′. . Epitheca showing 1′ and 6′′. . Antapical view showing Sp. Scale bars represent: 10 µm.