Figures & data
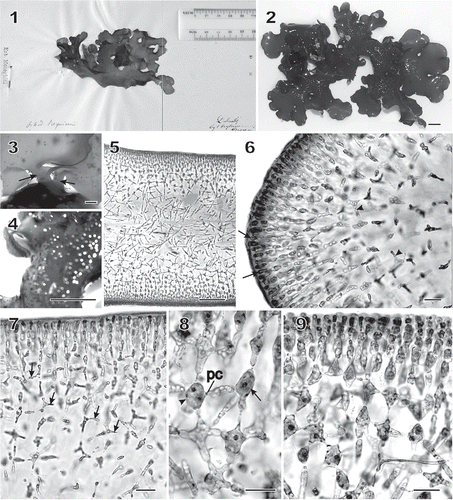
Figs 1–9 Chondrymenia lobata. Habit and vegetative morphology (Figs 2, 4–7, 9: HGI-A 6905; Fig. 3: HGI-A 6960; Fig. 8: HGI-A 2617). Figs 5–7, 9, aniline-blue stained; Fig. 8, haematoxylin stained. 1. Lectotype of Halymenia lobata in FI. 2. Typical lobed habit of a female gametophyte. 3. Two stipes (arrows) arising from a common discoid holdfast. 4. Protuberant cystocarps scattered on both surfaces of blade. 5. Transverse section near thallus margin. 6. Longitudinal section of multiaxial margin showing elongated apical initials (arrows), primary cortical and medullary filaments, and lateral connections between files of subcortical cells (arrowheads). 7. Transverse section near apex showing lateral connections between files of subcortical cells by means of horizontal arms with pit connections (arrows). 8. An enucleate horizontal arm-bearing cell (arrow) that has linked to the cell on the left (arrowhead) by means of a conjunctor cell depositing its nucleus and leaving a pit connection (pc). Other arms and pit connections give rise to stellate-shaped cells. 9. Transverse section showing files of cortical and subcortical filaments linked by lateral arms and secondary pit connections in a stellate arrangement. Scale bars = 1 cm (Figs 2, 4), 2000 µm (Fig. 3), 100 µm (Fig. 5) and 20 µm (Figs 6–9).
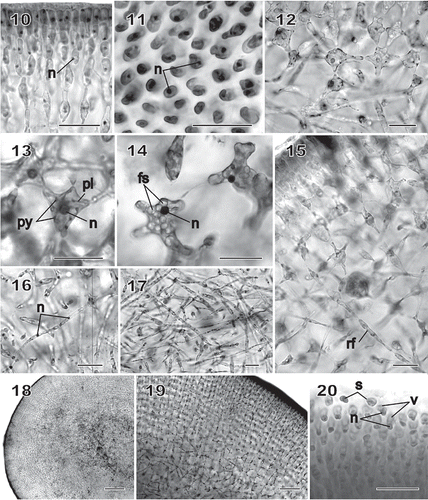
Figs 10–20 Chondrymenia lobata. Vegetative and spermatangial morphology (Figs 10, 12, 13, 16, 17: HGI-A 6905; Figs 11, 14, 18, 19: HGI-A 2617; Fig. 15: HGI-A 8384; Fig. 20: HGI-A 3267). Figs 10, 11, 14–16, 20, haematoxylin stained; Figs 12, 13, 17–19, aniline-blue stained. 10. Transverse section of outer cortical layer showing uninucleate (n) cells. 11. Uninucleate (n) outer cortical cells in surface view. 12. Transverse section at boundary between subcortical and outer medullary layers. 13. Subcortical cell with a nucleus (n) and a reticulate plastid (pl) and containing pyrenoid-like structures (py). 14. A uninucleate (n) subcortical cell densely filled with floridean starch grains (fs). 15. Rhizoidal filament (rf) growing towards the medulla through a latticework of subcortical cells. 16. Single nuclei (n) in cells of medullary filaments. 17. A dense weft of rhizoidal filaments intermixed with primary medullary filaments. 18. Transverse section of a stipe. 19. Enlarged view of stipe cortex showing cellular arrangement. 20. Spermatangia differentiating into and releasing uninucleate (n), vacuolate (v) spermatia (s) from surface of spermatangial sorus. Scale bars = 20 µm (Figs 10–17, 20), 200 µm (Fig. 18) and 50 µm (Fig. 19).
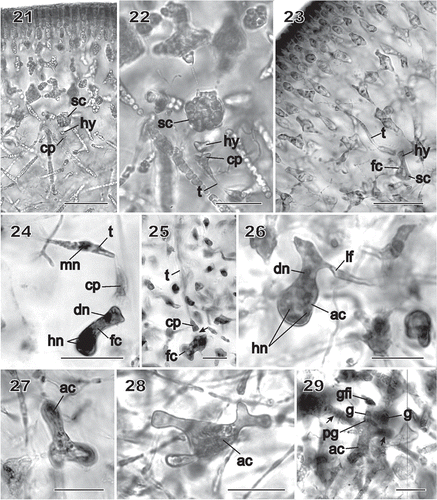
Figs 21–29 Chondrymenia lobata. Female-gametangia and early post-fertilization stages (Figs 21, 22: HGI-A 2617; Fig. 23: HGI-A 8384; Figs 24, 29: HGI-A 3267; Fig. 25: HEC 3692; Figs 26–28: HGI-A 6905). Figs 21–27, 29, haematoxylin stained; Fig. 28 aniline-blue stained. 21. Early pre-fertilization configuration of the supporting cell (sc), hypogynous cell (hy) and carpogonium (cp) near boundary between subcortex and medulla. 22. Enlarged view of procarp in Fig. 21; the carpogonium (cp) is terminated by an inwardly directed trichogyne (t). 23. Early post-fertilization stage showing a trichogyne remnant (t) and fusion cell (fc) presumed to have been formed by the fusion of the diploidized hypogynous cell (hy) with the supporting cell (sc). 24. A presumed male nucleus (mn) inside the trichogyne (t) and zygote nucleus (dn) in upper part of fusion cell (fc). 25. Stage similar to that in Fig. 24 showing a remnant cytoplasmic strand (arrow) between the carpogonium (cp) and the fusion cell (fc). 26. Auxiliary cell (ac) containing a large anterior diploid nucleus (dn) and several small posterior haploid nuclei (hn). A lateral filament (lf) issues from the distal end of the auxiliary cell. 27. Initiation of lobes from an originally saccate auxiliary cell (ac). 28. Lobed auxiliary cell (ac) that appears to be linked to gametophytic filaments. 29. Early stage of gonimoblast development. The auxiliary cell (ac) has cut off a gonimoblast initial, seen as the primary gonimoblast cell (pg) that has formed a cluster of gonimoblast cells (g) and a gonimoblast filament initial (gfi). Lateral filaments (arrows) can be seen emerging from opposite sides of the auxiliary cell. Scale bars = 20 µm (Figs 22, 24–29) and 50 µm (Figs 21, 23).
Figs 30–35 Chondrymenia lobata. Stages of gonimoblast and associated placental gametophyte development (Figs 30, 31, 34, 35: HGI-A 6905; Fig. 32: HGI-A 6960; Fig. 33: HGI-A 8384). Figs 30, 31, aniline-blue stained; Figs 32–35, haematoxylin stained. 30. Placental network of interconnected gametophytic filaments presumed to be associated with nearby gonimoblast formation, surrounded by a thick pericarp (p). 31. Enlarged cells with dense contents within the placental network. 32. Fusion of placental filaments with the auxiliary cell to produce a placental fusion cell (pfc) from which the gonimoblast filaments (gf) arise. 33. Gonimoblast filaments (gf) radiating towards the thallus surface from a gonimoblast fusion cell (gfc) that has fused onto the placental fusion cell (pfc). 34. Elongation of the placental fusion cell (arrow) through the apparent incorporation of cell lineages that originally bore the supporting cell. See also the two opposite lateral filaments (lf) originally cut off from the auxiliary cell, and the fusion of medullary cells belonging to the same branch lineage to form a long tubular ‘root’ that extends deep into the medulla (arrow). 35. Enlarged view of chains of uninucleate (n) carposporangia (c). Scale bars = 100 µm (Fig. 30), 20 µm (Figs 31–33, 35) and 50 µm (Fig. 34).
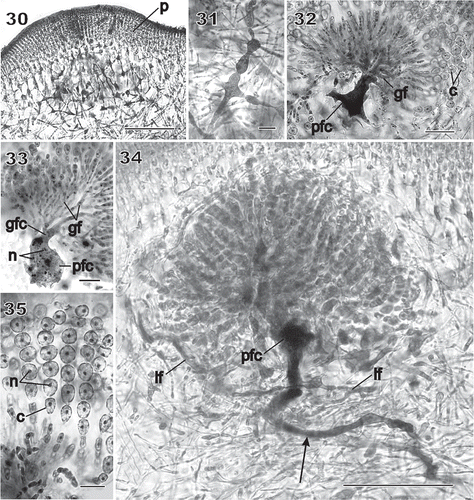
Figs 36–40 Chondrymenia lobata. Cystocarp (Fig. 36: HGI-A 2520; Figs 37–39: HGI-A 6905; Fig. 40: HGI-A 8384). Figs 36, 38–40, haematoxylin stained; Fig. 37, aniline-blue stained. 36. Immature cystocarp showing gonimoblast filaments (gf) radiating from a gonimoblast fusion cell (gfc) subtended by a placental fusion network (pfn) composed primarily of gametophytic filaments. 37. Cross-section of a mature cystocarp. The ostiolate (o) pericarp covers a chamber of carposporangial chains borne on a gonimoblast fusion cell (gfc) subtended by a placental fusion cell (pfc). 38. Apical region of a sinuous multinucleate lateral filament (lf) containing haploid nuclei (hn) spreading horizontally from the auxiliary cell through the inner cortex. 39. Anticlinal view of filaments forming the surface layers of the mature pericarp. 40. Detail of mature chains of carposporangia (c) internal to the pericarp (p). Scale bars = 100 µm (Figs 37, 38) and 20 µm (Figs 39–41).
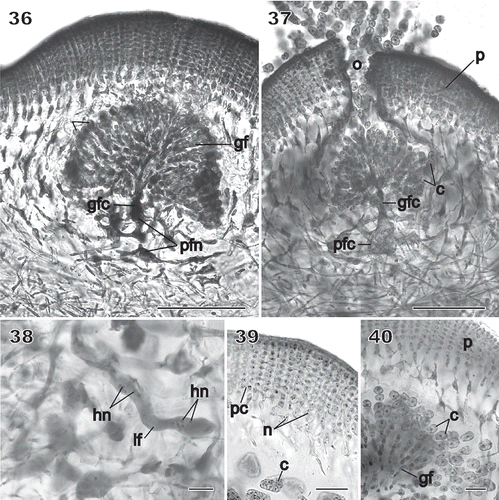
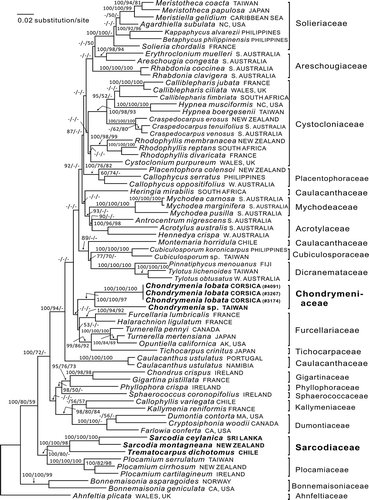
Fig. 41 RbcL phylogeny: ML tree (In L = −21877.5021) of the proposed new family Chondrymeniaceae from the Mediterranean Sea and north-western Pacific Ocean, compared with a selection of the related families. The numbers above the branches are the Bayesian posterior probabilities and the bootstrap values for the ML and the MP topology in that order. Dashes indicate less than 50% support values. The complex of families that include the Chondrymeniaceae are marked by an arrow on the left-hand side. Family names cited in the tree are from Guiry & Guiry (Citation2013), except that Callophycus is placed here in the Placentophoraceae.