Figures & data
Fig. 1. Map showing the distribution of Calliblepharis hypneoides along the Atlantic Iberian Peninsula. The black square indicates the type locality and black circles indicate the sites where material was collected for DNA analysis.
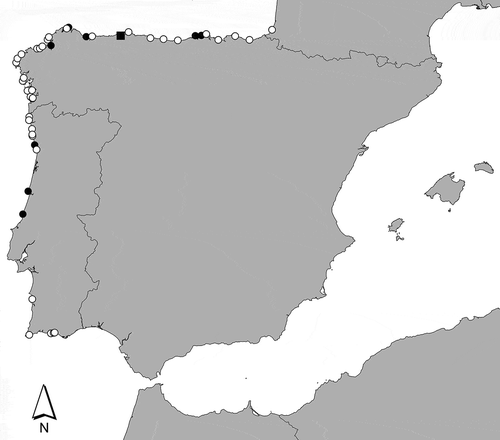
Figs 2–7. Calliblepharis hypneoides. Habit and habitat. 2. Type specimen collected in the Aguilar beach, Asturias, Spain, (SANT-Algae 26428). 3–7. Turf on sand-covered rocks from the lower intertidal at Biarritz, France (3, 7), the low intertidal at Aguilar beach, Asturias, Spain (4, 6); and the upper subtidal of Santa Comba beach, Galicia, Spain (5). Scale bar = 2 cm.
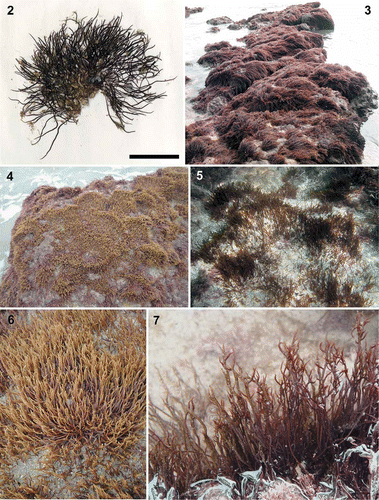
Figs 8–14. Calliblepharis hypneoides. Vegetative morphology. 8. Habit consisting of a fibrous basal system of entangled branches from which upright axes arise. 9. Peg-like projection of an axis of the basal system. 10. Branches connected by a lateral peg-like projection. 11. Vegetative upright axes sparsely and pseudodichotomously branched, that bear numerous spiniform proliferations in median and upper parts. 12. Upright axes with pseudodichotomous and apparent verticillate branchlets growing from a damaged part of the thallus. 13. Upright axes with opposite branches. 14. Upright axes with numerous alternate branchlets bearing tetrasporangial sori. Scale bars = 1 cm (Figs 8, 11), 200 µm (Fig. 9), 400 µm (Fig. 10) and 3 mm (Figs 12–14).
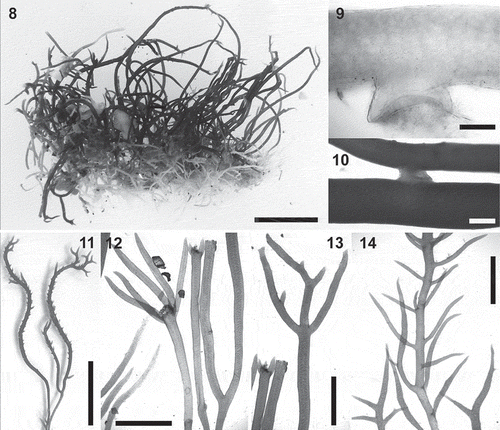
Figs 15–22. Calliblepharis hypneoides. Structure of the thallus. 15, 17. Longitudinal sections at apex, showing the apical cell of a uniaxial tip (a) and axial cells (arrowheads) that are soon obscured by the development of lateral files of cells. 16, 18. Longitudinal sections of median parts, showing two or three central filaments of elongated cells, surrounded by two or three layers of rounded medullary cells, a layer of short, rounded inner cortical cells, and a layer of small outer cortical cells. 19. Cross-section of axis showing a central axial cell surrounded by seven cells that are similar in appearance to the axial cell, two layers of medullary cells, a layer of inner cortical cells, and a layer of outer cortical cells. 20. Cross-section of axis with the central axial cell indistinguishable, resembling a pseudoparenchymatous medulla. 21. Surface view of thallus showing a continuous layer of outer cortical cells. 22. Detail of cortical cells in surface view showing spherical refractive inclusions (arrowheads). Scale bars = 30 µm (Fig. 15), 250 µm (Figs 16, 18), 60 µm (Fig. 17), 150 µm (Fig. 19), 100 µm (Fig. 20), 50 µm (Fig. 21) and 25 µm (Fig. 22).
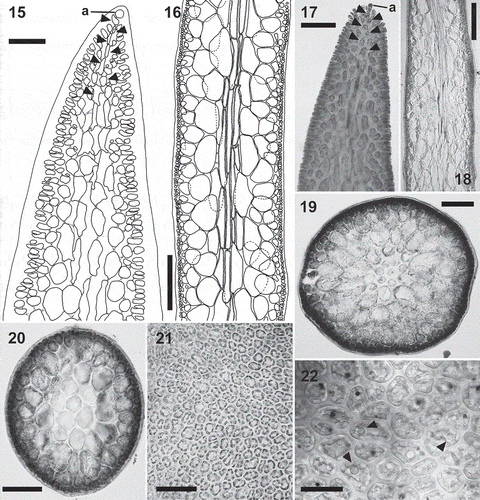
Figs 23–31. Calliblepharis hypneoides. Male reproductive structures. 23, 24. Surface view of spermatangial sorus surrounding upper branchlets. 25, 26. Cross-section of immature (Fig. 25) and mature (Fig. 26) surface spermatangial sori. 27. Primordium of spermatangial filaments after the first oblique division (arrow) and spermatangial filaments with a remnant basal rounded cell (arrowheads showing their pit connections to inner cortical cells). 28. Spermatangial primordium (arrowhead) after the first division leading to the formation of spermatangial filaments from the point of the first separated cell (arrows). 29. Spermatangial chains three or four cells long, which are simple (arrowhead) or branched (arrow). 30. Spermatangial filaments with one to three mature spermatia that have differentiated apical nuclei and basal vacuoles at the tip of each filament (arrowheads). 31. Sorus with mature spermatia and released spermatia with a central nucleus (arrowheads) and elongated cells (arrows), which correspond to primordia that have not cut off spermatangial filaments. Scale bars = 600 µm (Fig. 23), 70 µm (Figs 24, 26), 50 µm (Fig. 25) and 30 µm (Figs 27–31).
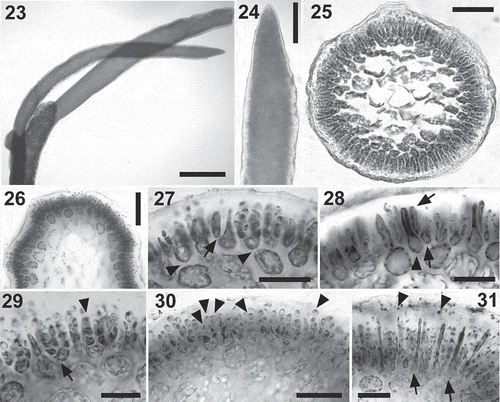
Figs 32–39. Calliblepharis hypneoides. Female stages. 32. Longitudinal section of an apical part of thallus showing the position of carpogonial branches (arrowheads). 33. Detail of a procarp, consisting of a supporting cell (su) bearing a three-celled carpogonial branch towards the inside (1–3), and an auxiliary cell (au). 34. Post-fertilization stage in longitudinal section, showing the supporting cell (su) bearing the disintegrating carpogonial branch (1–3) with the trichogyne elongating towards the thallus surface (arrowheads), gonimoblast initial (gi) cut off inwardly from the auxiliary cell (ac), modified cortical filaments (mcf) and the nutritive filaments (nf). 35. Cross-section showing an enlarged auxiliary cell (ac) and gonimoblast initial (gi), surrounded by modified cortical filaments (mcf) and nutritive filaments (nf). 36, 37. Gonimoblast initial (gi) forming ascending and descending gonimoblast filaments (agf, dgf), linked by secondary pit connections to nutritive filaments (nf). 38. Cross-section showing the auxiliary cell (ac) and the gonimoblast initial (gi) forming ascending (agf) and descending gonimoblast filaments (dgf) that link to basal nutritive filaments (nf). 39. Early branched gonimoblasts (g) growing from a reticulum (r) that consists of a meshwork of small, interconnected cells, and showing apical chains of 3–4 immature carpospores. Scale bars = 70 µm (Fig. 32), 20 µm (Figs 33–35, 37) and 50 µm (Figs 36, 38, 39).
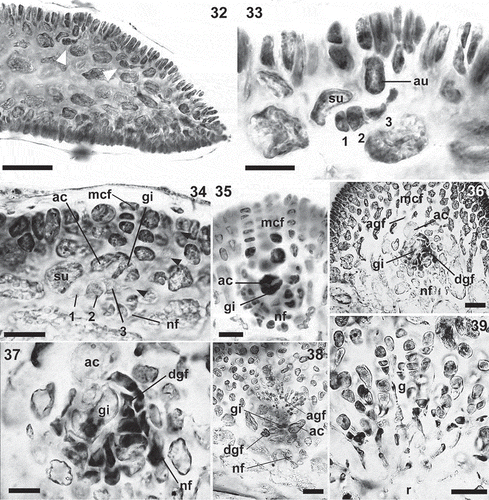
Figs 40–45. Calliblepharis hypneoides. Female structures. 40. Upper part of a female thallus with cystocarps. 41, 42. Cross-section of young cystocarps showing the basal cushion of nutritive filaments (nf), the central reticulum of interconnected cells (r) and the gonimoblasts (g) bearing carposporangia in chains. 43. Cross-section of a mature cystocarp showing the central reticulum surrounded by chains of carposporangia. 44. Cystocarp with a remnant cortical filament (arrowheads). 45. Chains of mature carpospores, with the outermost of them germinating inside the cystocarp. Scale bars = 3 mm (Fig. 40), 50 µm (Figs 41, 42, 44, 45) and 200 µm (Fig. 43).
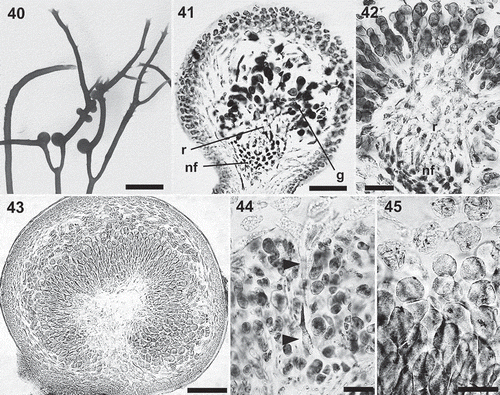
Figs 46–53. Calliblepharis hypneoides. Tetrasporangia. 46, 47. Surface view of tetrasporangial sorus surrounding the branchlets in upper regions of thallus. 48. Cross-section of a tetrasporangial sorus. 49. Longitudinal section of a tetrasporangial sorus showing a terminal tetrasporocyte (arrowhead) formed by the division of an outer cortical cell. 50. Longitudinal section of a tetrasporangial sorus showing an enlarged tetrasporocyte whose pit connection to its bearing cell shifting to a lateral position (arrowhead). 51. Longitudinal section of a tetrasporangial sorus showing a tetrasporocyte after its first division to form two cells, retaining the pit connection to its inner bearing cell (arrowhead). 52. Tetrasporocytes dividing to form tetraspores showing that the pit connection to its bearing cell (arrowheads) remains with the third cell counting from the outermost cell. 53. Cross-section of a tetrasporangial sorus showing two mature tetrasporangia zonately divided into four tetraspores. Scale bars = 700 µm (Fig. 46), 300 µm (Fig. 47), 100 µm (Fig. 48) and 30 µm (Figs 49–53).
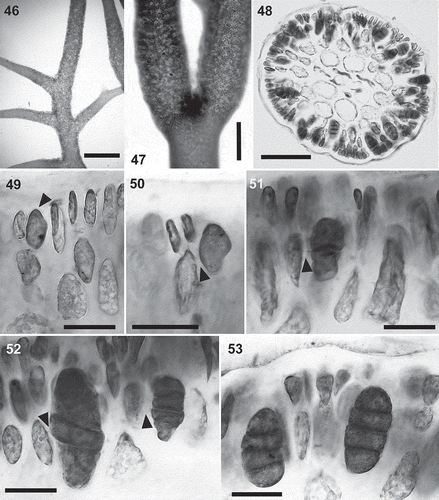
Fig. 54. The maximum likelihood phylogeny of Calliblepharis hypneoides based on rbcL data (–lnL = 8552.880744). Support values shown on or below branches are maximum likelihood (MLBt), maximum parsimony (MPBt) and Bayesian posterior probability (BPP) from DNA data.
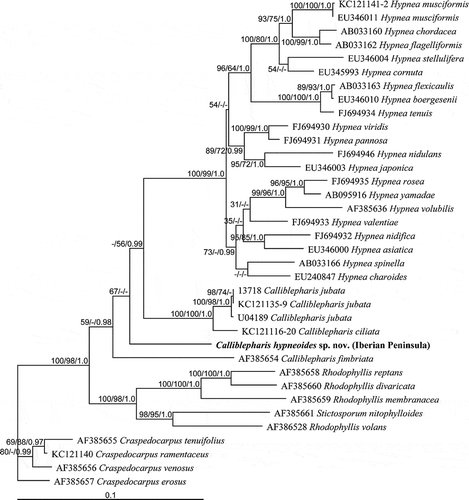
Fig. 55. The maximum likelihood phylogeny of Calliblepharis hypneoides based on SSU data (–lnL = 4706.262031). Support values shown on or below branches are maximum likelihood (MLBt), maximum parsimony (MPBt) and Bayesian posterior probability (BPP) from DNA data.
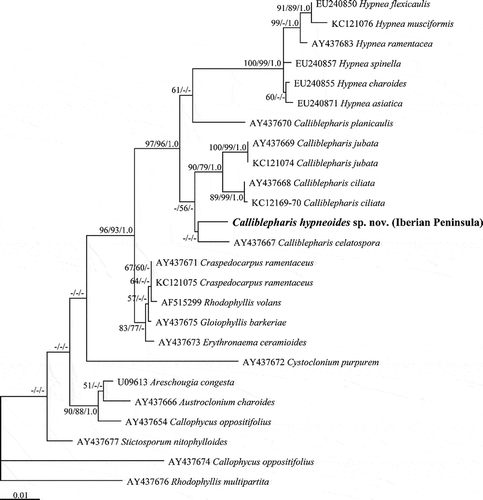
Table 1. Comparison of selected morphological characteristics in the species of the genus Calliblepharis.
Table 2. Comparison of selected morphological characteristics of genera of Cystocloniaceae.