Figures & data
Table 1. Origin and sampling details of analysed strains.
Fig. 1. Morphological features measured in Synura scales; scale length (sl), scale width (sw), base hole area (bha), keel pore area (kpa), base-plate-pore area (bpa), keel width (kw) and number of struts (sn). Scale bar represents 1 µm.
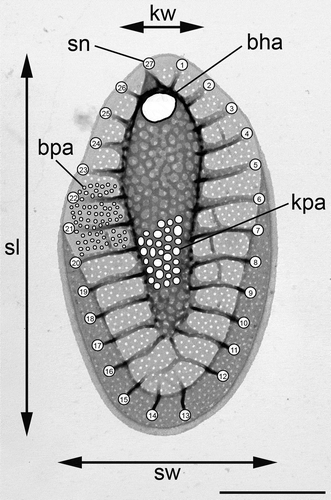
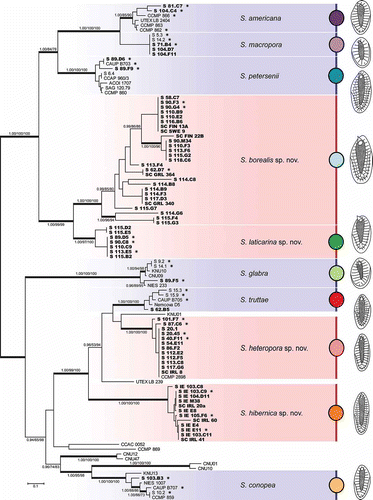
Fig. 2. Phylogeny of the genus Synura, section Peterseniae, obtained by Bayesian inference of the concatenated ITS rDNA, rbcL and cox1 dataset. The analysis was performed under a partitioned model, using different substitution models for each partition. Values at the nodes indicate statistical support estimated by three methods; MrBayes posterior node probability (left), maximum likelihood bootstrap (middle), and maximum parsimony bootstrap (right). Only statistical supports higher than 0.90/50/50 are shown. Thick branches highlight nodes receiving the highest posterior probability (PP) support (1.00). Newly sequenced strains are marked in bold. Strains used for the statistical analyses of morphological features are marked by asterisks. Scale bar represents the expected number of substitutions per site.
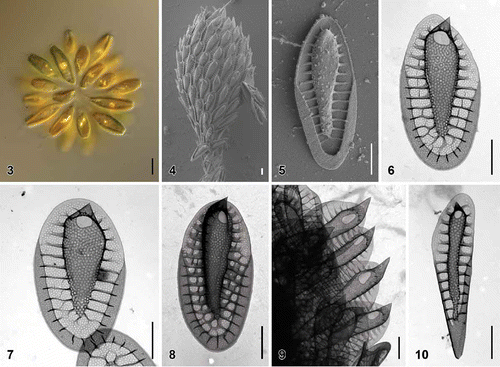
Figs. 3–10. Scale morphology of Synura borealis sp. nov. (Fig. 3: LM; Figs 4, 5: SEM; Figs 6–10: TEM). Scale bars represent 10 µm (Fig. 3) and 1 μm (Figs 4–10). Fig. 3. Colony consisting of elongated, lanceolate-shaped cells (strain S 90.G4). Fig. 4. Single cell surrounded by a layer of siliceous scales (S 90.G4). Fig. 5. Body scale (S 90.G4). Fig. 6. Body scale with transverse folds interconnecting the struts. Note the over-layered pore pattern at the scale keel (S 62.D7). Fig. 7. Body scale with obviously anteriorly widened keel (S 62.D7). Fig. 8. Apical scale (S 62.D7). Fig. 9. Apical scales with prominently protruding keel tips (S 58.C7). Fig. 10. Rear scale (S 62.D7).
Figs. 11–19. Scale morphology of Synura heteropora sp. nov. (Fig. 11: LM; Figs 12, 13, 16: SEM; Figs 14, 15, 17–19: TEM). Scale bars represent 10 μm (Fig. 11) and 1 μm (Figs 12–19). Fig. 11. Colony consisting of densely grouped, pyriform cells (strain S 20.45). Fig. 12. Single cell surrounded by a layer of siliceous scales (S 54.E11). Fig. 13. Body scale (S 54.E11). Fig. 14. Body scale (S 20.45). Fig. 15. Body scale with transverse folds interconnecting the struts (S 87.C6). Fig. 16. Apical scale (S 54.E11). Fig. 17. Apical scale with a pronounced, rounded keel tip (S 101.F7). Figs 18, 19. Rear scales (S 101.F7).
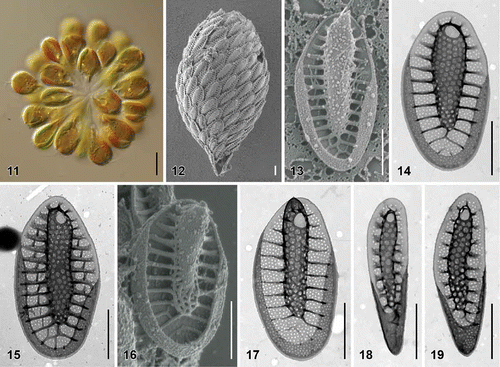
Figs. 20–28. Scale morphology of Synura hibernica sp. nov. (Fig. 20: LM; Figs 21, 22: SEM; Figs 23–28: TEM). Scale bars represent 10 μm (Fig. 20) and 1 μm (Figs 21–28). Fig. 20. Colony consisting of significantly elongated cells (strain S IE 104.D11). Fig. 21. Single cell surrounded by a layer of siliceous scales (S IE 104.D11). Fig. 22. Body scale (S IE 104.D11). Fig. 23. Body scale (S IE 103.C9). Fig. 24. Body scale (S IE E11). Fig. 25. Apical scale with prominently protruding keel tip (S IE E11). Fig. 26. Lateral view on apical scales with protruding keel tips (environmental sample, Lough Anillaun, Co. Galway, Ireland). Fig. 27. Rear scale (S IE M38). Fig. 28. Rear scale (S IE E11).
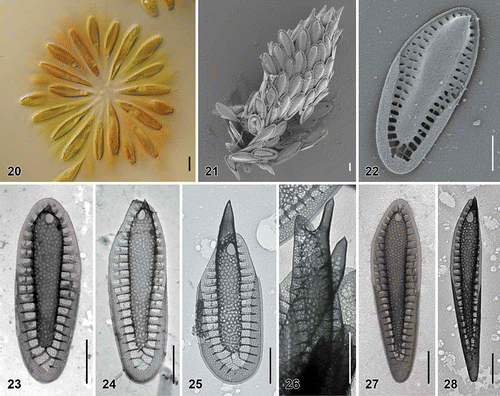
Figs. 29–36. Scale morphology of Synura laticarina sp. nov. (Fig. 29: LM; Figs 30 and 31: SEM; Figs 32–36: TEM). Scale bars represent 10 μm (Fig. 29) and 1 μm (Figs 30–36). Fig. 29. Colony consisting of pyriform cells (strain S 90.C8). Fig. 30. Single cell surrounded by a layer of siliceous scales (S 90.C8). Fig. 31. Body scale (S 90.C8). Fig. 32. Body scale with anteriorly widened keel (S 89.D5). Fig. 33. Body scale. Note over-layered pore pattern at the scale keel (S 89.D5). Fig. 34. Body scale with transverse folds interconnecting the struts (S 90.C8). Fig. 35. Apical scale (S 89.D5). Fig. 36. Rear scale (S 89.D5).
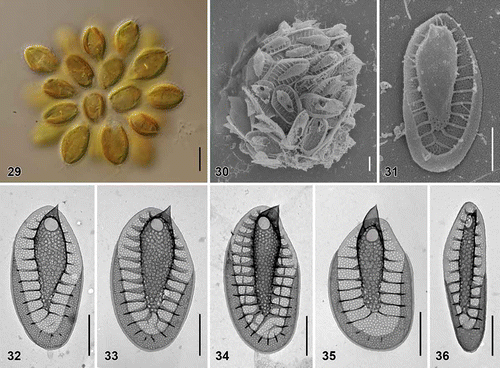
Figs. 37–42. Morphological comparisons and statistical analyses of siliceous scales. Figs 37–40. Scatterplots of morphological features measured in the 10 investigated species; average values and standard deviations are given. Fig. 37. Scatterplot of scale length versus scale width. Fig. 38. Scatterplot of base-plate pore area versus number of struts. Fig. 39. Scatterplot of base hole area versus keel pore area. Fig. 40. Scatterplot of keel pore to base-plate pore area ratio versus keel width. Fig. 41. Principal component analysis (PCA) of the entire measured morphological features dataset. Fig. 42. Canonical discriminant analysis (CDA) of the same dataset.
Table 2. Scale characteristics of selected Synura species (section Peterseniae) with similar scale morphologies. Newly described species are given in bold. For terminology of measured characteristics, see .
Table 3. Classification matrix of the canonical discriminant analyses of all seven morphological characters/three best discriminating characters (base-plate pore area, keel width, number of struts). Rows: observed classification. Columns: predicted classification.
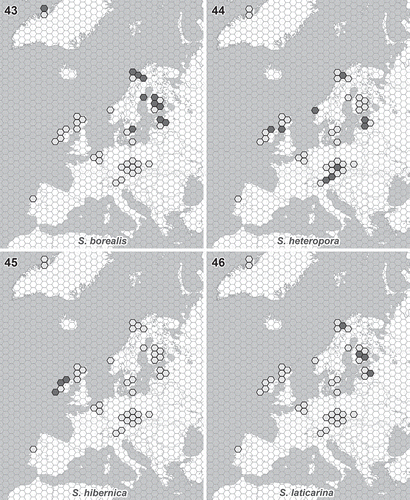
Figs. 43–46. Distribution of newly described Synura species. Light grey hexagons show all studied regions where the occurrence of any Synura species has been recorded. Filled hexagons show the known distribution pattern of the new species. Fig. 43. S. borealis. Fig. 44. S. heteropora. Fig. 45. S. hibernica. Fig. 46. S. laticarina. The hexagon edge length corresponds to 80 km (hexagon area ≈ 16 600 km2).