Figures & data
Fig. 1. Schematic diagram showing the biochemical reactions from squalene epoxide to cycloartenol and lanosterol. In plants, sterols are synthesized via the five ring intermediate, cycloartenol. In animals and fungi, sterols are made via the four ring intermediate lanosterol. Lanosterol synthase (ERG7) and cycloartenol synthase (CAS1) synthesize these reactions.
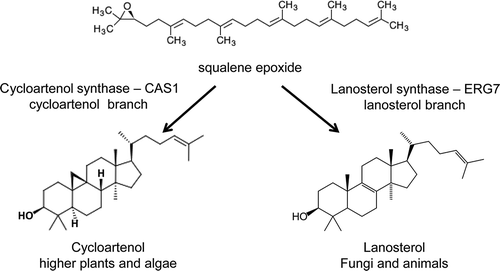
Table 1. Orthologues of sterol biosynthetic genes in Saccharomyces cerevisiae, Arabidopsis thaliana and Chlamydomonas reinhardtii.
Table 2. Chlamydomonas reinhardtii ergosterol genes and genome location.
Fig. 2. Structure of the CAS1 gene of Chlamydomonas reinhardtii and multiple sequence alignment of key amino acids in both lanosterol synthase and cycloartenol synthase across species. * = conserved residues. Amino acids 381, 449 and 453 are highly conserved residues, D455 is the catalytic residue. The numbering of the amino acids is based on the Homo sapiens sequence. The crystal structure of the oxidosqualene cyclase in H. sapiens has been published (Thoma et al., Citation2004).
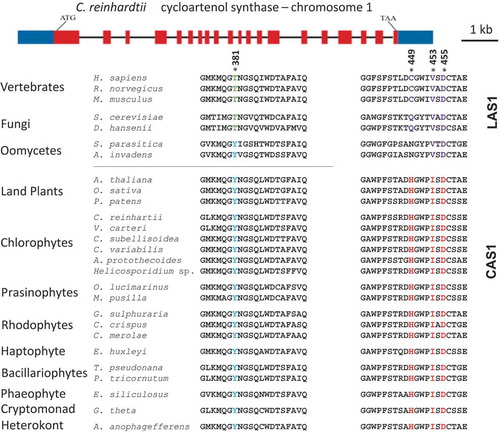
Table 3. Identification of CPI1 in other organisms.
Fig. 3. Structure of the CPI1 gene of Chlamydomonas reinhardtii and schematic diagram of the chemical conversion of cycloeucalenol to obtusifoliol. CPI1 helps open the cyclopropane ring of cycloeucalenol to form obtusifoliol.
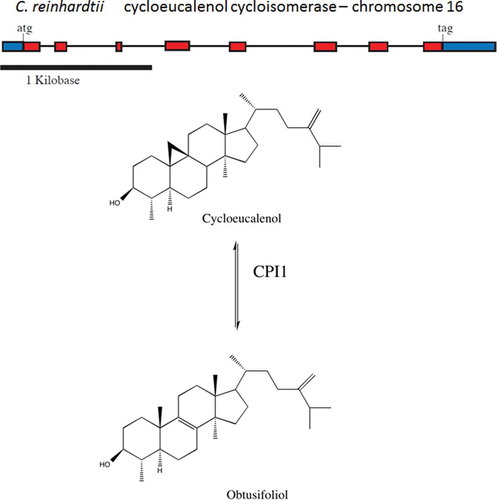
Table 4. Evidence for the expression of C. reinhardtii sterol biosynthetic genes.
Fig. 4. Transcript levels of sterol biosynthetic genes during reflagellation. Fold increase in expression for seven genes of the sterol biosynthetic pathway 1 hour after deflagellation compared with control cells that did not undergo deflagellation. The data are the mean (+/- standard error) of three biological replicates, each done in duplicate. The primers used in this qRT-PCR study are listed in Supplementary .
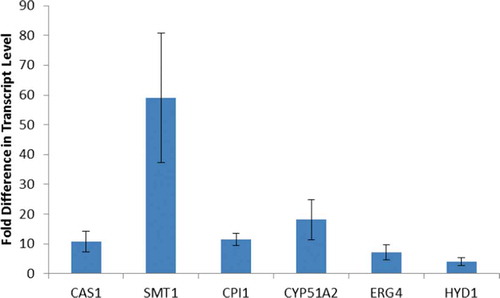
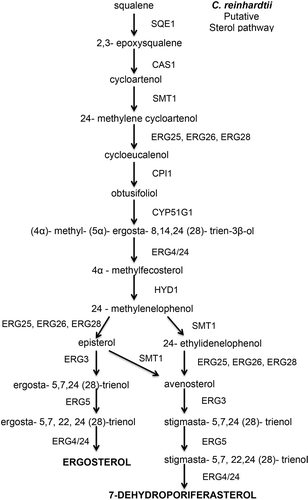
Fig. 5. Putative sterol pathway in Chlamydomonas reinhardtii showing the reactants and enzymes that catalyse the steps to synthesize ergosterol in C. reinhardtii.