Figures & data
Table 1. Comparison of selected morphological characteristics among the Polysiphonia sensu stricto 1 and 2, Vertebrata, Carradoriella, Streblocladia, ‘Polysiphonia’ schneideri and Melanothamnus clades.
Table 2. Genus Vertebrata with new combinations resulting from the present study printed in bold, followed by basionyms and taxonomic synonyms.
Table 3. Genus Vertebrata with resurrected names resulting from the present study and V. lanosa printed in bold, followed by basionyms and taxonomic synonyms.
Table 4. Genus Melanothamnus with new combinations resulting from the present study printed in bold, followed by basionyms and taxonomic synonyms.
Table 5. New combinations in Melanothamnus made for formal reasons (because the current genus is here placed in synonymy with Melanothamnus) although type material has not been examined. They are printed in bold, followed by basionyms and taxonomic synonyms.
Fig. 1. Phylogenetic tree estimated with ML analysis of rbcL sequences. Values at nodes indicate bootstrap support (BP)/posterior probability (PP) (only shown if > 60/0.6). Branches marked with an asterisk received 100% (BP)/1.00 (PP) support. Species names printed in bold correspond to type species of genera.
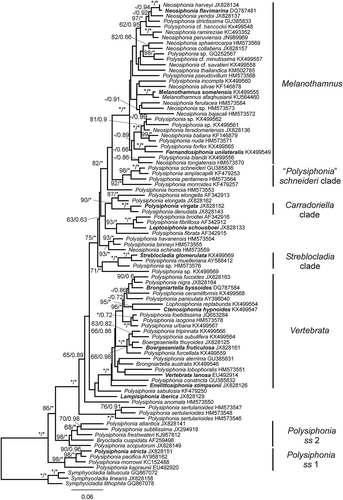
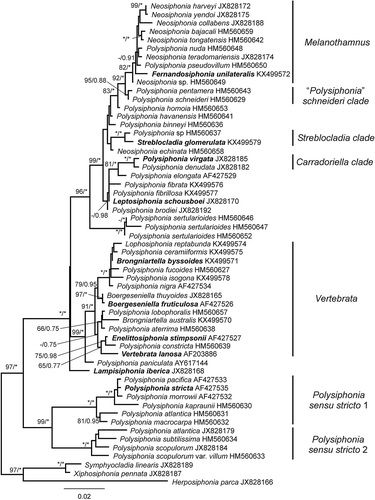
Fig. 2. Phylogenetic tree estimated with ML analysis of 18S sequences. Values at nodes indicate bootstrap support/posterior probability (only shown if > 60%/0.6 PP). Branches marked with an asterisk received 100%/1.00 PP support. Species names printed in bold correspond to type species of genera.
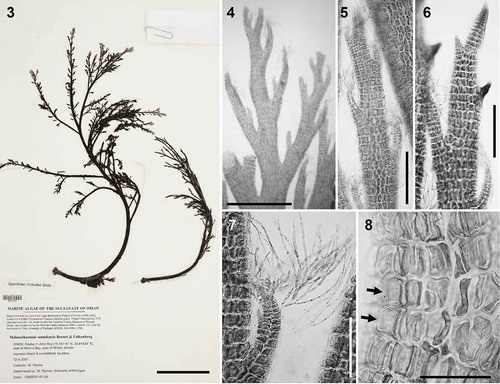
Figs 3–8. Melanothamnus somalensis, the type species of Melanothamnus. Fig. 3. Herbarium specimen MICH 662774. Fig. 4. Apical part of a specimen with alternately arranged branches. Figs 5–6. Apices of branches with (Fig. 5) or without (Fig. 6) abundant trichoblasts. Fig. 7. Apex of a lateral branch with trichoblasts. Fig. 8. Surface view of cells with the plastids lying exclusively on radial walls while the outer walls appear transparent (arrows). Scale bars: Fig. 3, 6 cm; Fig. 4, mm; Figs 5 and 6, 350 µm; Fig. 7, 200 µm; Fig. 8, 100 µm.
Figs 9–18. Fernandosiphonia unilateralis type material, the type species of Fernandosiphonia. Fig. 9. Herbarium specimen. Figs 10–11. Branches unilaterally arranged. Fig. 12. Axis with scar cells of trichoblasts (arrows). Figs 13–14. Surface view of pericentral cells with plastids lying only on the radial walls, so that the outer walls appear transparent (Fig. 13, arrows) and cells have a dark flank (Fig. 14). Fig. 15. Young spermatangial branch formed on the first dichotomy of a trichoblast, the other branch remaining vegetative (arrow). Fig. 16. Procarp (su = supporting cell; cp = carpogonium). Fig. 17. Cystocarp. Fig. 18. Tetrasporangia arranged in short spiral series. Scale bars: Fig. 9, cm; Fig. 10, mm; Fig. 11, 450 µm; Figs 12, 14, 17 and 18, 100 µm; Figs 13 and 15, 40 µm; Fig. 16, 20 µm.
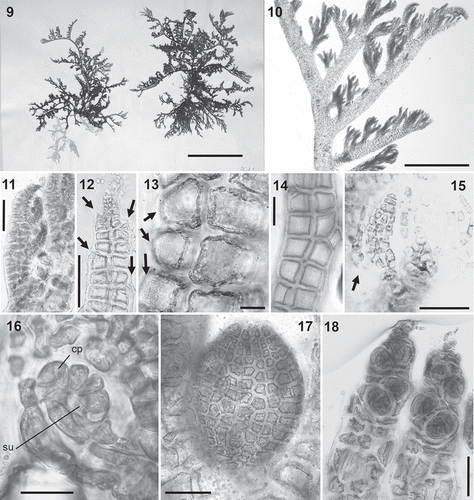
Figs 19–24. Rhizoid anatomy in the Polysiphonieae. In open connection with pericentral cells in Polysiphonia stricta (Fig. 19, Polysiphonia sensu stricto clade 1). Cut off from pericentral cells in P. foetidissima (Fig. 20, Vertebrata clade), P. denudata (Fig. 21, Carradoriella clade), Polysiphonia sp. (Fig. 22, Streblocladia clade), P. schneideri (Fig. 23, ‘P.’ schneideri clade) and P. incompta (Fig. 24, Melanothamnus clade). Scale bars: Figs 19–23, 100 µm; Fig. 24, 500 µm.
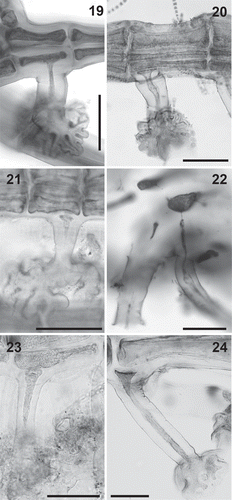
Figs 25–39. Plastid arrangement in the Polysiphonieae. Scattered against all cell walls of the pericentral cells in Polysiphonia stricta (Figs 25–26, Polysiphonia sensu stricto clade 1), Vertebrata lanosa (Figs 27–28, Vertebrata clade), P. virgata (Figs 29–30, Carradoriella clade), Polysiphonia sp. (Fig. 31, Streblocladia clade) and P. schneideri (Figs 32–33, ‘P.’ schneideri clade). Lying exclusively on the radial walls of the pericentral cells in species of the Melanothamnus clade: Neosiphonia collabens (Figs 34–35), N. harveyi (Figs 36–38) and P. forfex (Fig. 39). Scale bars: Figs 25, 27, 29, 38 and 39, 500 µm; Figs 26, 28 and 30, 800 µm; Figs 31, 32, 34, 35 and 37, 100 µm; Fig. 33, 300 µm; Fig. 36, 50 µm.
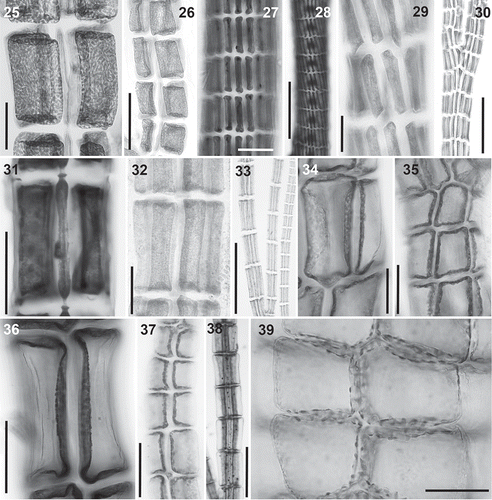
Figs 40–46. Trichoblast nuclei (arrows) in the Polysiphonieae. Uninucleate trichoblast cells in Polysiphonia scopulorum (Fig. 40, Polysiphonia sensu stricto clade 1), P. denudata (Fig. 44, Carradoriella clade), P. schneideri (Fig. 45, ‘P.’ schneideri clade) and P. blandii (Fig. 46, Melanothamnus clade). Multinucleate trichoblast cells in species of the Vertebrata clade: P. nigra (Fig. 41), Boergeseniella fruticulosa (Fig. 42) and P. foetidissima (Fig. 43). Scale bars: Figs 40–43, 60 µm, Fig. 44, 30 µm; Fig. 45, 20 µm; Fig. 46, 100 µm.
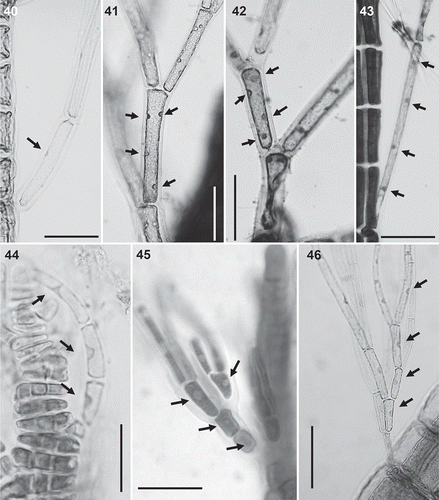
Figs 47–52. Spermatangial branches in the Polysiphonieae. Replacing trichoblasts and with sterile apical filaments in Polysiphonia stricta (Fig. 47, Polysiphonia sensu stricto clade 1). Replacing trichoblasts and lacking sterile apical cells in Vertebrata lanosa (Fig. 48, Vertebrata clade). On a branch of a trichoblast and with sterile apical cells in P. fucoides (Fig. 49, Vertebrata clade), P. denudata (Fig. 50, Carradoriella clade), P. schneideri (Fig. 51, ‘P.’ schneideri clade) and Neosiphonia harveyi (Fig. 52, Melanothamnus clade). Scale bars: 100 µm. Arrows show the apical sterile cells and arrowheads the sterile branch of fertile trichoblasts.
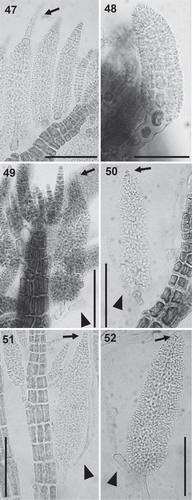
Figs 53–58. Carpogonial branches in the Polysiphonieae. Four-celled in Polysiphonia stricta (Fig. 53, Polysiphonia sensu stricto clade 1), P. nigra (Fig. 54, Vertebrata clade), P. denudata (Fig. 55, Carradoriella clade) and P. schneideri (Fig. 56, ‘P.’ schneideri clade). Three-celled in species of the Melanothamnus clade: Neosiphonia harveyi (Fig. 57) and P. blandii (Fig. 58). Su = supporting cell; st = sterile basal cell; 1–4 cells of carpogonial branches. Scale bars: Fig. 53, 30 µm; Figs 54–58, 20 µm.
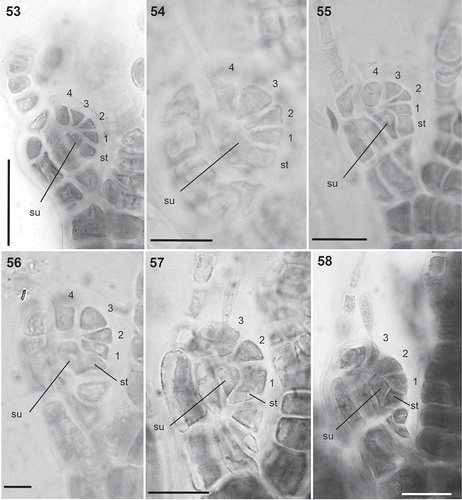
Figs 59–64. Cystocarps in the Polysiphonieae. Urceolate in Polysiphonia stricta (Fig. 59, Polysiphonia sensu stricto clade 1). Ovoid in Vertebrata lanosa (Fig. 60, Vertebrata clade), P. denudata (Fig. 61, Carradoriella clade), Streblocladia glomerulata (Fig. 62, Streblocladia clade). Globose in Polysiphonia schneideri (Fig. 63, ‘P.’ schneideri clade) and Neosiphonia collabens (Fig. 64, Melanothamnus clade). Scale bars: Figs 59–62 and 64, 200 µm; Fig. 63, 100 µm.
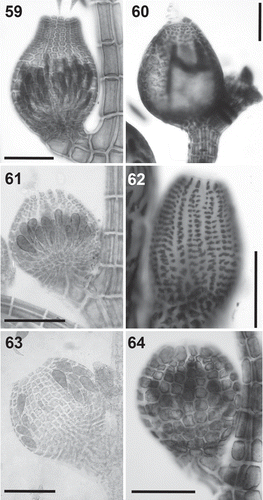
Figs 65–70. Cells surrounding the ostiole in the Polysiphonieae. Similar or slightly larger than the cells of the pericarp inmediately below in Polysiphonia stricta (Fig. 65, Polysiphonia sensu stricto clade 1), Vertebrata lanosa (Fig. 66, Vertebrata clade), P. denudata (Fig. 67, Carradoriella clade), and P. schneideri (Fig. 69, ‘P.’ schneideri clade). They are much larger in Streblocladia glomerulata (Fig. 68, Streblocladia clade) and Neosiphonia collabens (Fig. 70, Melanothamnus clade). Scale bars: Figs 65–68 and 70, 100 µm; Fig. 69, 60 µm.
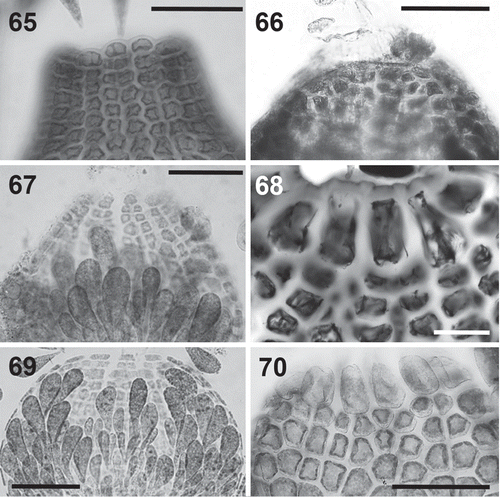
Figs 71–76. Tetrasporangia in the Polysiphonieae. Forming long straight series in Polysiphonia stricta (Fig. 71, Polysiphonia sensu stricto clade 1). Forming spiral series in Vetebrata lanosa (Fig. 72, Vertebrata clade), Polysiphonia sp. (Fig. 74, Streblocladia clade) and Neosiphonia harveyi (Fig. 76, Melanothamnus clade). Forming short straight series in P. denudata (Fig. 73, Carradoriella clade), and P. schneideri (Fig. 75, P. schneideri clade). Scale bars: Figs 71, 74 and 76, 200 µm; Figs 72, 73 and 75, 400 µm.
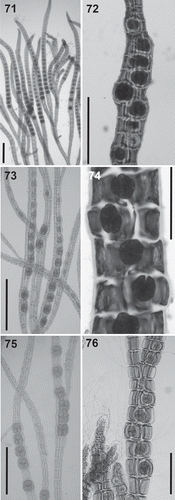
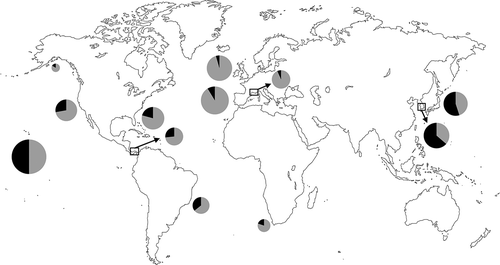
Fig. 77. World map representing the proportion of Melanothamnus (black) and other Polysiphonieae (grey) species in selected regions where the Polysiphonieae were studied in detail. Data were obtained from the following references after updating the species names: Alaska: Lindstrom (http://www.seaweedsofalaska.com); Brazil (Espírito Santo-São Paulo): Guimâraes et al. (Citation2004); California: Abbott & Hollenberg (Citation1976); Florida: Schneider & Searles (Citation1991); Hawaii: Abbott (Citation1999); Japan: Yoshida (Citation1998); Korea: Nam & Kang (Citation2012); Panama: Mamoozadeh & Freshwater (Citation2012); South Africa: Stegenga et al. (Citation1997); southern France: Lauret (Citation1967, Citation1970) Spain (Galicia): Bárbara et al. (Citation2005); British Isles: Maggs & Hommersand (Citation1993).