Figures & data
Fig. 1. Maximum likelihood phylogeny of the Halymeniales based on the rbcL gene using the Tamura 3-parameter + GAMMA model in MEGA6 (–Ln = 11385.4511). Support values resulting from the Maximum likelihood, Maximum parsimony and Bayesian analyses are indicated as branch labels (ML/MP/BI). Scale indicates substitutions per site
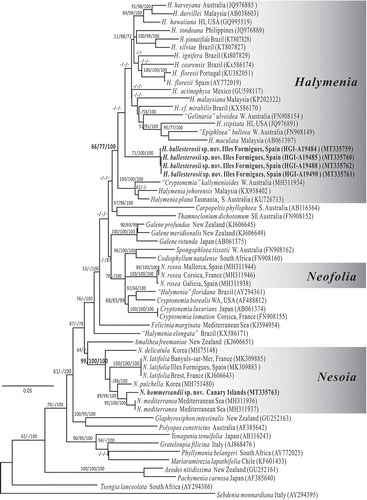
Figs 2–13. Halymenia ballesterosii Rodríguez-Prieto, S.-M. Lin, De Clerck & Huisman sp. nov. Thallus morphology, vegetative structure, sporophyte and male reproductive structure. Fig. 2. Holotype, a monoecious specimen with cystocarps scattered over fertile thallus surface (HGI-A 19484). Fig. 3. Isotype, a female gametophyte consisting of a bifurcating and apically eroded blade (HGI-A 19490). Fig. 4. Cross section of a young blade showing ovoid surface cells (black arrows), ovoid subcortical pit-connected cells (black arrowhead at right), third layer of stellate cells with short arms (white arrowhead), inner cortical cell with long arms (black arrowhead at left) and anticlinally arranged medullary filament (white arrows) (HGI-A 19490). Fig. 5. Surface view of a blade showing rounded to irregularly polygonal cortical cells with reticulate plastid (arrows) (HGI-A 19484). Fig. 6. Close up of a cross section of the blade showing ovoid and protruding surface cells (arrows) (HGI-A 19499). Fig. 7. Subsurface view of thallus blade showing subcortical ovoid to slightly stellate cells with very short arms. Note that cells are secondarily pit connected (arrows) and bear a reticulate plastid (pl) (HGI-A 19490). Fig. 8. Subsurface view of thallus blade showing the inner subcortical cells layer, composed of stellate cells with quite long arms (arrows) (HGI-A 11077). Fig. 9. Subsurface view of thallus blade showing the network of medullary stellate cells with long arms (arrows). Note that the network is composed both by darkly staining (black arrows) and non-darkly staining medullary cells (white arrows) (HGI-A 6552). Fig. 10. Detail of the medulla and medullary stellate cells of Fig. 9 (HGI-A 6552). Fig. 11. Subsurface view showing a tetrasporangial initial (arrowhead) and many scattered tetrasporangial initials that are divided once (arrows) (HGI-A 19488). Fig. 12. Cross section through a tetrasporangial sorus showing cruciately divided tetrasporangium (arrow) (HGI-A 19498). Fig. 13. Surface view of spermatangial sori (arrows) (HGI-A 19477). Haematoxylin (Figs 4, 5); Aniline blue (Figs 6–12); Not stained (Fig. 13). Scale bars: Figs 2, 3 = 1 cm; Figs 4, 5, 7, 12, 13 = 20 µm; Figs 6, 10, 11 = 10 µm; Fig. 8 = 50 µm; Fig. 9 = 100 µm
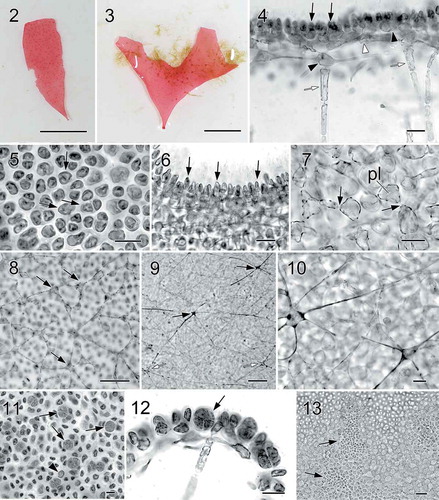
Figs 14−23. Halymenia ballesterosii Rodríguez-Prieto, S.-M. Lin, De Clerck & Huisman sp. nov. Morphology of carpogonial branch and auxiliary cell ampullae and development of cystocarp. Fig. 14. Close up of a carpogonial branch ampulla showing a 2-celled carpogonial branch with a short trichogyne and short branched ampullar filament (af) borne on a subcortical cell (arrow) (HGI-A 19485). Fig. 15. Early post-fertilization of carpogonial branch ampulla showing a 2-celled carpogonial branch with a fertilized carpogonium (cp) and a well-developed distally swelled trichogyne (tri). Note that the cells of carpogonial branch and the cells of the ampullar filaments cut off lateral branches (arrowheads) and the enlarged basal cell (black arrow) fused with neighbouring cells to form a fusion cell and became darkly stained. Few small derived cells (= nutritive cells, white arrows) were cut off from the basal part of the fusion cell (HGI-A 11077). Fig. 16. A later stage of Fig. 15, showing the carpogonial branch (cb) and the fusion cell (black arrow) cutting off more nutritive cells/filaments (white arrows) (HGI-A 11077). Fig. 17. A young auxiliary cell ampulla showing ampullar filaments (af) and the auxiliary cell (aux) (HGI-A 19490). Fig. 18. A fully developed auxiliary cell ampulla showing the three orders of branched ampullar filaments (af1, af2 & af3) and auxiliary cell (aux) (HGI-A 19490) Fig. 19. Early stage after diploidization showing two primary gonimoblast cells (g) just cut off from the gonimoblast initial (gi) borne on the newly formed fusion cell (fc), flanking ampullar filaments (black arrows), and two secondarily produced connecting filaments (cf’). Note that the ampullar cells close to the fusion cell (white arrows) enlarged slightly (HGI-A 19490). Fig. 20. A slightly later stage of Fig. 19, showing primary gonimoblasts (g) cut off from the gonimoblast initial (gi) borne on fusion cell (fc), cells of ampullar filaments (white arrows), and a secondarily produced connecting filament (cf’) (HGI-A 19490). Fig. 21. Immature carposporophyte showing gonimoblast initial (gi), two gonimoblasts (g1 & g2) borne on a small fusion cell (fc), and a secondarily produced connecting filament (cf’). Note that most cells (white arrows) of ampullar filament near the fusion cell are darkly stained but do not branch further (HGI-A 19490). Fig. 22. Cross section of a fully developed cystocarp showing gonimoblasts (g) borne on a conspicuous basal fusion cell (fc) and ampullar filaments forming a filamentous pericarp (arrows) (HGI-A 19484). Fig. 23. Subsurface view of cystocarp-bearing blade showing gonimoblasts (g) and one of the mature cystocarps composed of two gonimolobes (g1 & g2) (HGI-A 19499). Aniline blue (Figs 14–21, 23); Not stained (Fig. 22). Scale bars: Figs 14, 16–22 = 20 µm; Figs 15, 23 = 50 µm
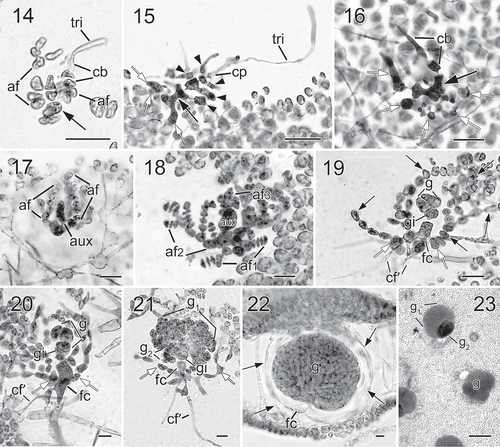
Figs 24–38. Nesoia hommersandii Rodríguez-Prieto, S.-M.Lin, Afonso-Carrillo, De Clerck & Huisman sp. nov. Thallus morphology, vegetative, and reproductive structure. Fig. 24. Holotype, a sporophyte with a single, simple blade and a short stipe (arrow) (TFC Phyc 15296). Figs 25–26. Isotypes, sporophytes bearing lobed margins or bladeletes (TFC Phyc 15297 and 15294). Fig. 27. Isotypes, a small monoecious gametophyte (arrowhead) attached to the basal part of a big sporophyte (arrow). Note that the gametophyte bears many cystocarps that can be distinguished at naked eye (= red dots) (TFC Phyc 15300). Fig. 28. Transverse section of a young blade showing a thin cortex, subcortical cell (arrowhead) and a large medulla with anticlinal medullary filaments (arrow). Note that plastids (pl) are network-like (TFC Phyc 15300). Fig. 29. Transverse section of another young blade showing a thin cortex and broadened medullary filaments (arrow) (TFC Phyc 15300). Fig. 30. Cross section of a young blade showing flattened surface cells (white arrows), ovoid subcortical pit-connected cells with short arms (arrowheads), and inner cortical cell with long arms (black arrow) (TFC Phyc 15300). Fig. 31. Polygonal outer cortical cells in surface view (TFC Phyc 15300). Fig. 32. Subsurface view of thallus blade showing stellate cells of medullary network (arrows) interconnected by long and thin arms (TFC Phyc 15301). Fig. 33. Transverse section showing cells of the anticlinal medullary filaments (arrow) (TFC Phyc 15300). Fig. 34. Transverse section of an old blade showing many rhizoidal filaments (arrows) within the medulla (TFC Phyc 15301). Fig. 35. Subsurface view of a tetrasporangial sorus showing immature tetrasporangia (arrows) and fully divided tetrasporangia (arrowheads) (TFC Phyc 15301). Fig. 36. Cross section through a tetrasporangial sorus showing immature tetrasporangia (arrows) and nearly mature tetrasporangia (arrowhead) (TFC Phyc 15301). Fig. 37. Close up of immature tetrasporangia (arrows) and mature tetrasporangia (arrowhead) (TFC Phyc 15300). Fig. 38. Subsurface view of a spermatangial sorus showing mature spermatangia (arrows) (TFC Phyc 15300). Haematoxylin (Figs 28, 36, 37). Aniline blue (Figs 29–35, 38). Scale bars: Figs 24–27 = 5 cm; Figs 28, 30, 33, 34, 35, 37, 38 = 20 µm; Figs 29 = 50 µm; Figs 31, 32, 36 = 10 µm
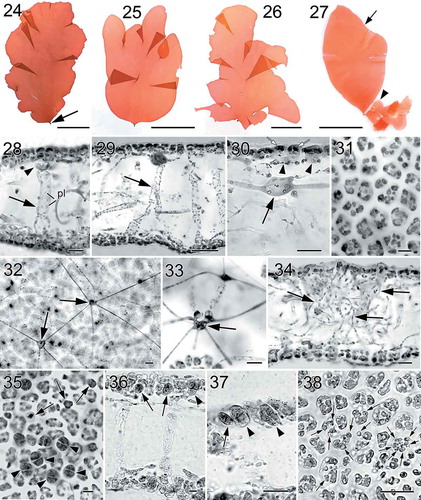
Figs 39–46. Nesoia hommersandii Rodríguez-Prieto, S.-M.Lin, Afonso-Carrillo, De Clerck & Huisman sp. nov. Morphology of carpogonial branch and auxiliary cell ampullae and cystocarp development. Fig. 39. Close up of a carpogonial branch ampulla showing a young carpogonial branch (cb) prolonged by a short trichogyne (tri) surrounded by short branched ampullar filaments (af) (TFC Phyc 15300). Fig. 40. Subsurface view of a fully developed carpogonial branch (cb) ampulla showing an enlarged trichogyne (tri) and highly branched ampullar filaments (af) (TFC Phyc 15300). Fig. 41. Close up of a developing auxiliary cell ampulla showing ampullar filaments (af) and the auxiliary cell (aux) (TFC Phyc 15300). Fig. 42. A fully developed auxiliary cell ampulla showing connecting filament (cf), ampullar filaments (af) and an enlarged auxiliary cell (aux) with stained nuclei (n). Note that a secondary connecting filament (cf’) is cut off from the basal part of the auxiliary cell (aux) (TFC Phyc 15300). Fig. 43. Diploidized auxiliary cell ampulla showing a gonimoblast initial (gi), a secondary connecting filament (cf’) cut off from basal part of newly formed fusion cell (fc) (TFC Phyc 15300). Fig. 44. Close up of a young carposporophyte showing an elongated gonimoblast initial (gi) and a fusion cell (fc) that cut off several secondarily connecting filaments (cf’) (TFC Phyc 15300). Fig. 45. An immature carposporophyte showing a gonimoblast initial (gi) and a fusion cell (fc) which is sending out two secondarily connecting filaments (cf’) (TFC Phyc 15300). Fig. 46. A fully developed carposporophyte showing fusion cell (fc) and two gonimolobes (g1 & g2) (TFC Phyc 15300). Aniline blue (Figs 39, 40, 44, 46); Haematoxylin (Figs 41–43, 45). Scale bars: Figs 39, 40 = 10 µm; Figs 41–45 = 20 µm; Fig. 46 = 50 µm
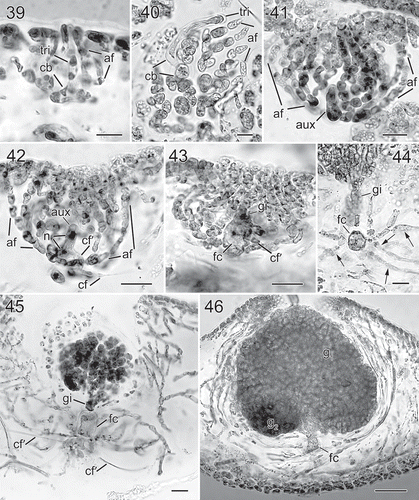
Table 1. Morphological comparison among foliose species of Halymenia, Neofolia and Nesoia from Europe
