Figures & data
Fig. 2. Maximum likelihood tree based on the DNA sequences of chloroplast rbcL and tufA gene, and nuclear 18S, 5.8S, 28S rDNA and ITS regions (total 5551 bp). Numbers on the branches indicate the bootstrap values (BP, right) and Bayesian posterior probabilities (PP, left). Only the BP (≥70%) and PP (≥0.90) are shown
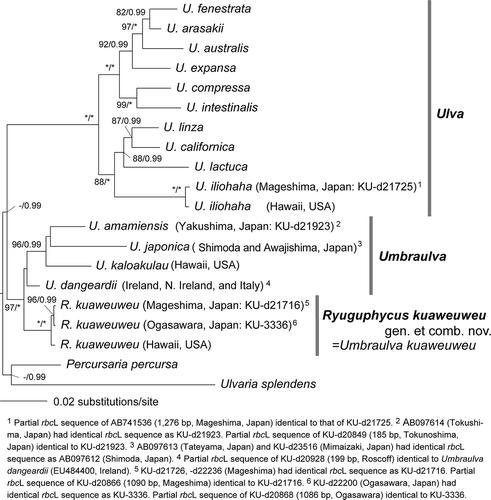
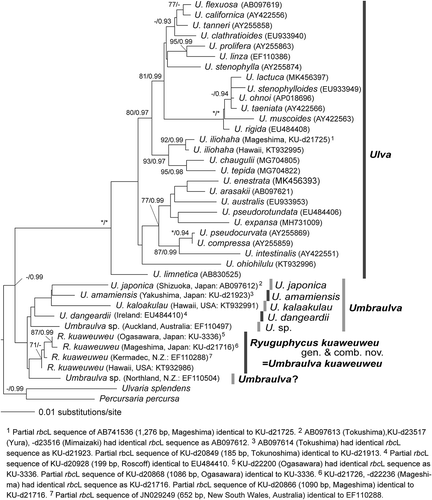
Fig. 3. Maximum likelihood (ML) molecular phylogeny based on DNA sequences of chloroplast rbcL genes (1327 bp). Numbers on the branches indicate the bootstrap values (BP, right) and Bayesian posterior probabilities (PP, left). Only the BP (≥70%) and PP (≥0.90) are shown
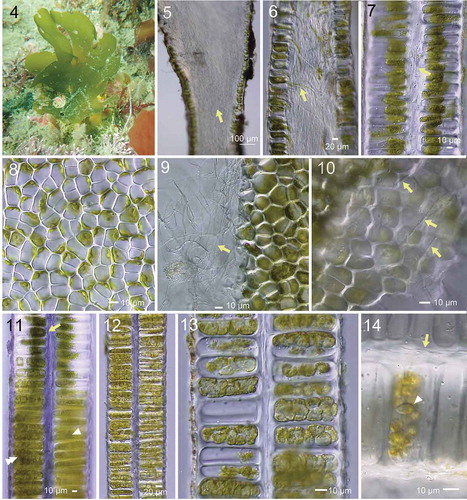
Figs 4–14. Morphology of Umbraulva japonica. Fig. 4. Habit of thallus (at ~5 m depth; 7 cm high) at Oishizaki, Awajishima, Japan. Figs 5, 6. Longitudinal section near the base. Note dense rhizoidal filaments (arrow) inside the thallus. Fig. 7. Longitudinal section of upper part of thallus with inner rhizoidal filaments (arrow). Fig. 8. Surface view of thallus showing roundish, quadrate and polygonal cells. Fig. 9. Edge of torn thallus showing fine inner rhizoidal filaments (arrow). Fig. 10. View of interior side of surface cells exposed by tearing the thallus, showing fine rhizoidal filaments running parallel from base to distal parts of thallus (arrows). Fig. 11. Longitudinal section of thallus with different developmental stages of zoidangia. Arrow, premature. Arrowhead, condensation of cytoplasm. Double arrowhead, cytokinesis in developing zoidangia. Figs 12, 13. Developing zoidangia showing different developmental stages. Fig. 14. Mature and emptied zoidangia showing rhizoidal filaments (arrow) and mature zoid (arrowhead)
Figs 15–20. Morphology of Ryuguphycus kuaweuweu gen. et comb. nov. in crude culture. Fig. 15. Habit of young thallus with basal terete portion. Fig. 16. Lower terete part of thallus. Fig. 17. Inner rhizoidal filaments near the basal part of thallus (arrowheads). Fig. 18. Surface view of upper part of thallus. Each cell contains a parietal chloroplast with prominent pyrenoid (arrowhead). Fig. 19. Cross section of marginal portion of middle thallus with inner cavity. Fig. 20. Cross section of solid middle thallus
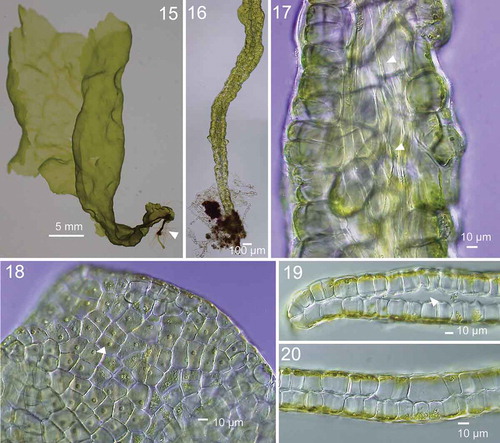
Figs 21–31. Development of thallus of Ryuguphycus kuaweuweu gen. et comb. nov. in unialgal culture. Figs 21, 22. Germlings. Figs 23, 24. Young biseriate erect thallus. Figs 25, 26. Young parenchymatous erect thalli. Fig. 27. Cross section of upper part of thallus showing hollow portion (arrowheads). Fig. 28. Cross section of mature zoidangia. Arrowheads show developing release pores. Fig. 29. Surface view of mature zoidangia in surface view. Note the remarkable size difference between large (arrowheads) and normal (arrows) zoids. Fig. 30. Mature zoidangia (arrowhead) and in situ germination of zoids (arrow) in surface view. Fig. 31. Surface view showing release pore (arrowheads) of zoidangia
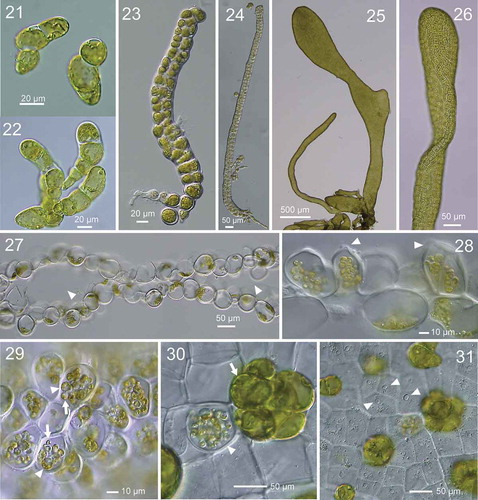
Figs 32–37. Development of zoidangia in Ryuguphycus kuaweuweu gen. et comb. nov. Figs 32–34. DNA epifluorescence microscopy. Figs 32a, 33a and 34a: Brightfield. Figs 32b, 33b and 33b: Epifluorescence. Fig. 32. Cross section of vegetative thallus. Note parietal chloroplasts located in the cell periphery along the thallus surface (Fig. 32a) and nuclei located on opposite sides of the cell (arrowheads in Fig. 32b). Fig. 33. Surface view of thallus during zoidangial development. Note nuclei at late anaphase (arrowhead) in small lobes of cytoplasm. Fig. 34. Surface view of mature zoidangia. Note a number of zoids with condensed nucleus within zoidangium (arrowhead). Figs 35, 36. Transmission electron micrograph of thin section of mature zoidangium. Fig. 35. Zoidangium with zoids with centripetal arrangement. Arrowhead indicates a flagellum of a zoid. Fig. 36. Higher-magnification view of a zoid in mature zoidangium showing a basal body (arrowhead) and an eyespot (arrow). Fig. 37. Light micrograph of released zoids with flagella (arrowheads)
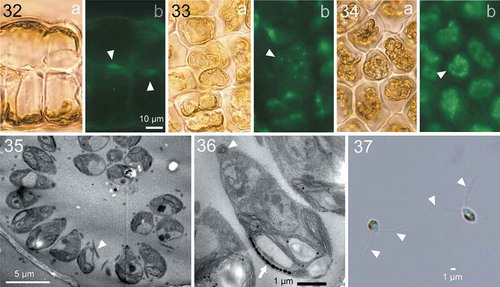
Fig. 38. HPLC chromatographs comparing accessory photosynthetic pigments among Ulva australis, Umbraulva japonica and Ryuguphycus kuaweuweu gen. et comb. nov. (= U. kuaweuweu). Note that the carotenoid siphonaxanthin is present in U. japonica and R. kuaweuweu but absent in Ulva australis. In contrast, R. kuaweuweu lacks α- and β-carotene, which are present in Ulva australis and Umbraulva kuaweuweu
Table 1. Comparison of habitat and selected taxonomic features between Ulva spp., Umbraulva represented by generitype U. japonica, and Ryuguphycus kuaweuweu gen. et comb. nov. (= Umbraulva kuaweuweu)