Figures & data
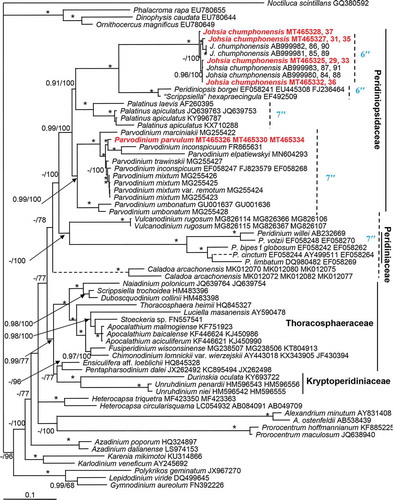
Figs 1–9. Micrographs of Johsia chumphonensis strain TIO606. Fig. 1. An empty theca showing the rupture (arrow) and a newly released athecate cell. Fig. 2. A dividing cell. Fig. 3. A small coccoid cell. Fig. 4. An empty small coccoid cell. Fig. 5. A spherical cyst showing numerous granules and an accumulation body (arrow). Fig. 6. Cyst showing paratabulation. Fig. 7. Ventral view showing a rounded epitheca and hypotheca with a prominent eyespot (arrow). Fig. 8. Dorsal view showing a ring like pyrenoid (P) and a round nucleus (N). Fig. 9. Epifluorescence image of a cell in ventral view showing the single reticulate chloroplast in the periphery of the cell. (Figs 1–5, 7–9) light microscopy. (Fig. 6) scanning electron microscopy. Scale bars = 5 μm
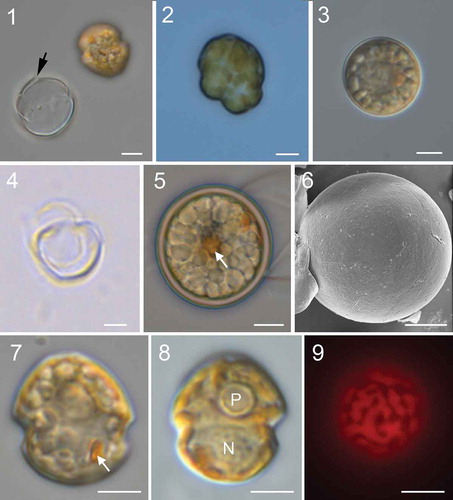
Figs 10–15. Scanning electron micrographs of vegetative cells of Johsia chumphonensis strain TIO606. Fig. 10. Ventral view showing the first apical plate (1′), anterior sulcal plate (Sa), posterior sulcal plate (Sp), and three cingular plates (C1, C2, C6). Fig. 11. Apical view showing four apical plates, two anterior intercalary plates (1a, 2a) and six precingular plates (1′′–6′′). Fig. 12. Internal apical view showing pore plate and canal plate (X). Fig. 13. Dorsal view showing two anterior intercalary plates, three precingular plates, three cingular plates and two postcingular plates (3′′′, 4′′′). Fig. 14. Antapical view showing five postcingular plates and two antapical plates (1′′′′, 2′′′′) of similar size. Fig. 15. Sulcal area showing Sa plate, right sulcal plate (Sd), left sulcal plate (Ss), and Sp plate. Scale bars = 5 μm
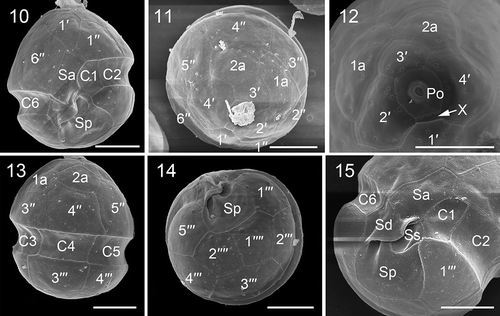
Figs 16, 17. Transmission electron micrographs of vegetative cells of Johsia chumphonensis strain TIO606. Fig. 16. Longitudinal sections through the cell showing a large nucleus (N), a stalked pyrenoid (P), and chloroplast lobes in the periphery of the cell (C). Fig. 17. The eyespot (e) located within a chloroplast lobe comprising two rows of globular lipids with elongated crystals overlying (arrow), neighbouring a nucleus with many chromosomes (Cr). Scale: Fig. 16 = 5 μm; Fig. 17 = 1 μm
Figs 18–23. Scanning electron micrographs of vegetative cells of Parvodinium parvulum strain TIO879. Fig. 18. Ventral view showing the first apical plate, anterior sulcal plate (Sa), and three cingular plates (C1, C2, C6). Fig. 19. Dorsal view showing two anterior intercalary plates (1a, 2a), three precingular plates (3′′–5′′), three cingular plates (C3–C5) and third postcingular plate (3′′′). Fig. 20. Apical view showing three apical plates (2′–4′), two anterior intercalary plates and six precingular plates (1′′–6′′). Fig. 21. Apical view showing four apical plates, pore plate (Po), cover plate (cp), canal plate (X) and apical pore. Fig. 22. Antapical view showing five postcingular plates (1′′′– 5′′′), two antapical plates (1′′′′, 2′′′′) of unequal size and numerous papillae (arrows). Fig. 23. Sulcal area showing Sa plate, Sd plate, Ss plate, Sm plate and Sp plate. Scale bars = 5 μm
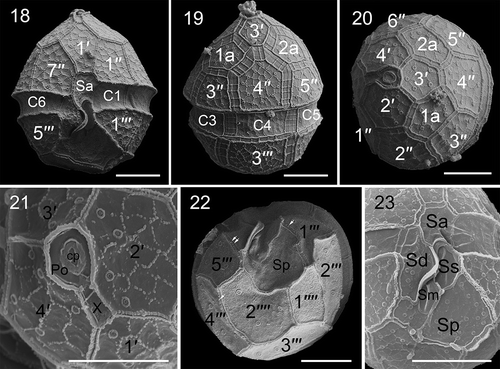
Fig. 24. Phylogeny of Johsia chumphonensis and Parvodinium parvulum inferred from concatenated SSU, ITS-5.8S and partial LSU rRNA (D1–D6) sequences using Bayesian inference (BI). New sequences are indicated in red. Branch lengths are drawn to scale, with the scale bar indicating the number of nucleotide substitutions per site. Numbers on branches are statistical support values to clusters on the right of them (left: Bayesian posterior probabilities (BPP); right: maximum likelihood (ML) bootstrap support (BS) values). Bootstrap support values > 50% and Bayesian posterior probabilities above 0.9 are shown. Asterisk indicates maximal support (ML BS = 100% and BPP = 1.00)