Figures & data
Figure 1. Sequence alignment of the predicted helix 7 for the hGLUT proteins and 3D map of hydrophobic residues lining the exofacial vestibule of GLUT7. Panel A: Sequences were aligned using Clustal X software and written from N to C-termini. Based on the putative membrane topology of this helix, its C-terminus end faces the extracellular side of the membrane. Numbers refer to the amino acid positions in the respective GLUT isoforms. Panel B: 3D model of hGLUT7 showing the position of hydrophobic residues at the opening of the exofacial vestibule. The model was created using RasMol and the structural model obtained using the coordinates from the crystal structure of Glp T and Lac Y.
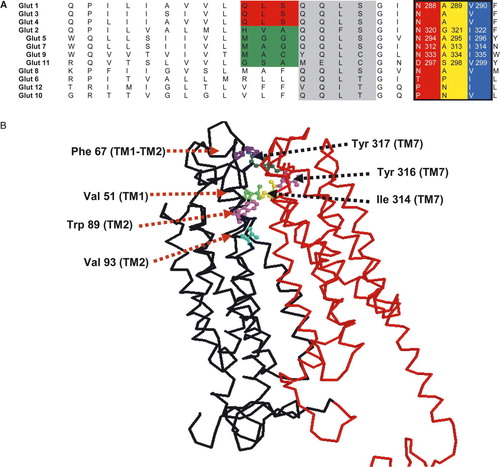
Figure 2. Kinetics of glucose transport in the WT and I296V GLUT5 mutant. [14C] D-Glucose uptake (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with mRNA (20 ng). Data represent mean uptake into 10–12 individual oocytes corrected for uptake into water-injected oocytes from a representative experiment. Curves were fitted by non-linear regression for a single Michaelis-Menten component with the kinetic constants for the WT (○) and I296V (•) of Kms = 0.63±19 and 0.36±0.09 mM, and Vmaxes = 22.7±2.0 and 18.14±1.01 pmol, oocytes−1, 30min−1, respectively. The fitted curves for the two sets of data were not significantly different (ANOVAR p>0.05).
![Figure 2. Kinetics of glucose transport in the WT and I296V GLUT5 mutant. [14C] D-Glucose uptake (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with mRNA (20 ng). Data represent mean uptake into 10–12 individual oocytes corrected for uptake into water-injected oocytes from a representative experiment. Curves were fitted by non-linear regression for a single Michaelis-Menten component with the kinetic constants for the WT (○) and I296V (•) of Kms = 0.63±19 and 0.36±0.09 mM, and Vmaxes = 22.7±2.0 and 18.14±1.01 pmol, oocytes−1, 30min−1, respectively. The fitted curves for the two sets of data were not significantly different (ANOVAR p>0.05).](/cms/asset/a999e2cf-b9e5-41c8-aadc-6f474eac89e6/imbc_a_229718_f0002_b.gif)
Figure 3. Substrate specificity of WT hGLUT9 and the effect of the mutation I335V. Uptake of [14C] labelled hexoses (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with WT hGLUT9 mRNA (20 ng), panel A or I335V mutant mRNA, panels B & C. In all cases data represent mean uptake into 10–12 individual oocytes corrected for uptake into water-injected oocytes from a representative experiment. Cytochalasin B (CB) was added at a concentration of 100 µM. Error bars represent the standard error of the mean. For the kinetic analysis of glucose uptake into oocytes expressing I335V GLUT9 shown in panel C a curve was fitted by nonlinear regression for a single Michaelis-Menten component with a Km of 0.116±mM and a Vmax of 7.2±pmol, oocytes−1, 30 min−1.
![Figure 3. Substrate specificity of WT hGLUT9 and the effect of the mutation I335V. Uptake of [14C] labelled hexoses (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with WT hGLUT9 mRNA (20 ng), panel A or I335V mutant mRNA, panels B & C. In all cases data represent mean uptake into 10–12 individual oocytes corrected for uptake into water-injected oocytes from a representative experiment. Cytochalasin B (CB) was added at a concentration of 100 µM. Error bars represent the standard error of the mean. For the kinetic analysis of glucose uptake into oocytes expressing I335V GLUT9 shown in panel C a curve was fitted by nonlinear regression for a single Michaelis-Menten component with a Km of 0.116±mM and a Vmax of 7.2±pmol, oocytes−1, 30 min−1.](/cms/asset/9dc885e2-c567-4241-97a1-195006d3eca4/imbc_a_229718_f0003_b.gif)
Figure 4. Kinetics of glucose and fructose transport in GLUT11A wild type. [14C] D-Hexose uptake (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with mRNA (20 ng). Data represent mean uptake into 10–12 individual oocytes corrected for uptake into water injected oocytes from a representative experiment which was repeated three times. Curves were fitted by non-linear regression for a single Michaelis-Menten component with a Km of 0.116±mM and a Vmax of 7.2±pmol, oocytes−1, 30 min−1 for glucose (panel A) and Km of 60±µM and a Vmax of 11±pmol, oocyte−1, 30min−1 for fructose (panel B). In each case the experiments were repeated three times with very similar results.
![Figure 4. Kinetics of glucose and fructose transport in GLUT11A wild type. [14C] D-Hexose uptake (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with mRNA (20 ng). Data represent mean uptake into 10–12 individual oocytes corrected for uptake into water injected oocytes from a representative experiment which was repeated three times. Curves were fitted by non-linear regression for a single Michaelis-Menten component with a Km of 0.116±mM and a Vmax of 7.2±pmol, oocytes−1, 30 min−1 for glucose (panel A) and Km of 60±µM and a Vmax of 11±pmol, oocyte−1, 30min−1 for fructose (panel B). In each case the experiments were repeated three times with very similar results.](/cms/asset/b16e91d9-7c62-4126-b191-627d1775163f/imbc_a_229718_f0004_b.gif)
Figure 5. Glucose and fructose transport mediated by GLUT11 wild type and the mutants D297N, S298A, V299I, and those with DSV replaced with NAI or NAV. Stage 5–6 Xenopus oocytes were injected with WT hGLUT11 or mutant mRNA (20 ng) or water 3 days before determinations of uptake of [14C] D-fructose or D-glucose (100 µM). Bars represent mean net uptake (30 min at 22°C) into 10–12 oocytes injected with mRNA corrected for the uptake into water injected oocytes under identical conditions. Panel A shows a representative experiment which was performed twice. Panel B shows a typical experiment for fructose and glucose uptake into oocytes expressing WT, NAI or NAV replacing the entire DSV motif. These experiments were repeated five times.
![Figure 5. Glucose and fructose transport mediated by GLUT11 wild type and the mutants D297N, S298A, V299I, and those with DSV replaced with NAI or NAV. Stage 5–6 Xenopus oocytes were injected with WT hGLUT11 or mutant mRNA (20 ng) or water 3 days before determinations of uptake of [14C] D-fructose or D-glucose (100 µM). Bars represent mean net uptake (30 min at 22°C) into 10–12 oocytes injected with mRNA corrected for the uptake into water injected oocytes under identical conditions. Panel A shows a representative experiment which was performed twice. Panel B shows a typical experiment for fructose and glucose uptake into oocytes expressing WT, NAI or NAV replacing the entire DSV motif. These experiments were repeated five times.](/cms/asset/587b9b63-8238-439f-a06c-d1cb7282358f/imbc_a_229718_f0005_b.gif)