Figures & data
Figure 1. Stereo representation of the monomer of Amt-1 from A. fulgidus (PDB accession code). Eleven α-helices cross the membrane. The protein is displayed in cartoon representation, with the protein chain coloured from blue at the N-terminus on the extracellular side (top) to red at the C-terminus on the cytoplasmic side (bottom).
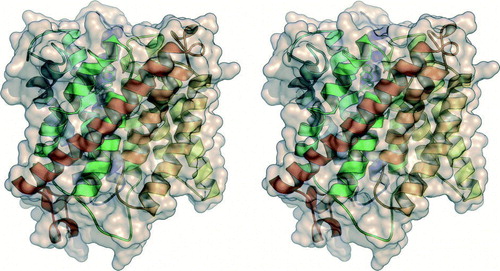
Figure 2. Substrate recruitment and transport in Amt-1. (A) A putative recruitment site for is located on the extracellular side of each Amt-1 monomer, where a water molecule was found in the model (red dot). The combination of hydrogen-bonding to Ser 208 and cation-π-interaction with Trp 137 provides selectivity. (B) Trimer of Amt-1 seen from the extracellular side. While each monomer is able to transport substrate, the trimeric arrangement is essential for interaction with the regulatory PII protein (). (C) A channel crosses the Amt-1 monomer, starting immediately below the putative substrate recruitment site at Typ 137 and Ser 208, extracellular side above, cytoplasmic side below. (D) Detail of (C). Substrate is blocked from passing through the channel by the side chains of Phe 96 and Phe 204, but once this obstacle is passed a channel leads directly to the cytoplasmic side of the membrane. It is lined exclusively by hydrophobic residues, with the exception of the conserved histidines His 157 and His 305.
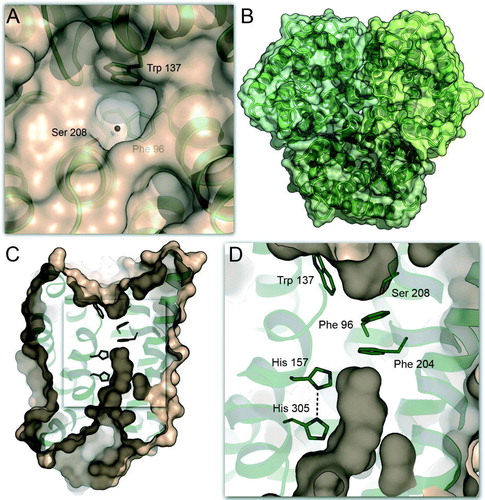
Figure 3. The complex of E. coli AmtB with GlnK. (A) Side view of the complex (PDB accession-# 2NS1) with the AmtB trimer shown in surface representation and GlnK as cartoon. In each trimer, one monomer is shown in colour. (B) Detail of the blocking of the cytoplasmic exit channel of AmtB (green) by GlnK (blue). The side chain of Arg 47 inserts into the channel, and Tyr 51, the site of uridylylation though GlnD, is in close proximity: (not shown).
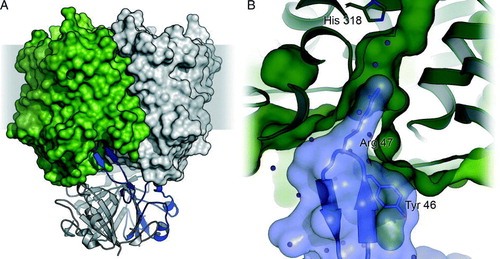
Figure 4. Mechanistic scheme of Amt transport. (A) At physiological pH values ammonium will be almost exclusively in the cationic form, such that translocation of NH3 implies extracellular deprotonation and intracellular reprotonation. Uniport of NH3 must therefore be considered a net antiport of versus H+. (B) Electrogenic transport as observed in some plant AMTs and Rhesus proteins is either a net uniport of
or a symport of NH3/H+.