Figures & data
Figure 1. TARP protein structure. Schematic representation of the secondary structure of TARP proteins, comprising four transmembrane domains and cytosolic carboxy and amino termini. Amino acid sequence of the cytosolic C-terminal tail aligned in TARPγ2, γ3, γ4 and γ8. Red letters indicate phosphorylated serine residues and blue letters, phosphorylated threonine residues. This Figure is reproduced in colour in Molecular Membrane Biology online.
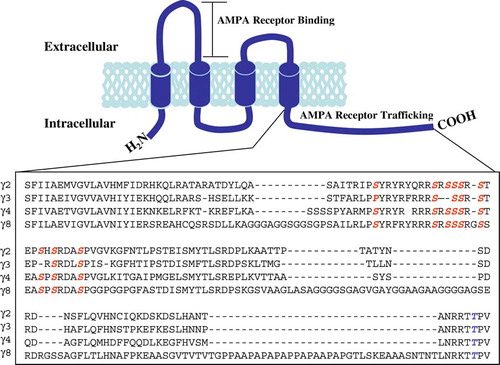
Figure 2. Immunoaffinity purification of TARPγ2 and its associated AMPAR subunits. (A) Immunoblot analysis of TARPγ2 protein throughout the purification assay. Immunoblot probed with an anti-TARPγ2 antibody raised to the extreme C-terminus. Input is cerebellar membranes from control (+/+:+/stg) and stargazer (stg) mice. Insol is the proportion of +/+:+/stg and stg material that remained in the insoluble fraction following Triton X-100 solubilization. FT is the flow-through the column, the soluble material that did not bind to the immunoaffinity column. Output, pH eluted fractions 1–5 are the purified material acid-eluted from the immunoaffinity column. (B) GluR2 solubilization and co-purification with TARPγ2 in control (+/+:+/stg) material. (C) GluR4 solubilization and co-purification with TARPγ2 in control (+/+:+/stg) material.
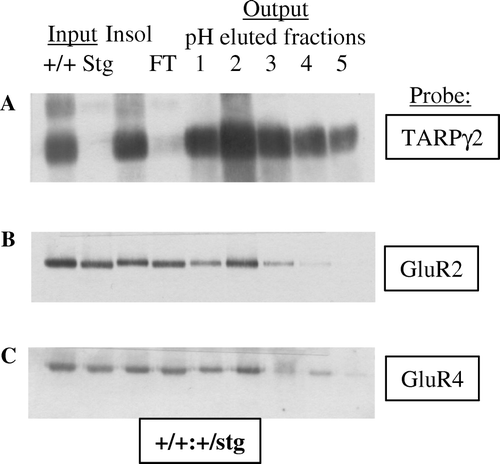
Table I. TARP isoforms.
Figure 3. AMPAR trafficking at the synapse. Two monomeric AMPAR subunits form a dimer (1), followed by the association of two dimers of AMPAR subunits to form a tetramer (2) in the endoplasmic reticulum (ER). TARPs associate with the tetrameric AMPAR to act as an auxiliary subunit (3) permitting the efficient export of the AMPAR from the ER to the Golgi (4). nPIST binds to the C-terminal tail of TARPγ2 in the Golgi (5) and acts to chaperone the AMPAR complex to the cell surface in vesicles (6). The TARPγ2-AMPAR complex diffuses into the PSD where PSD-95 binds to the PDZ binding domain of the C-terminal tail of TARPγ2 to anchor the complex at the synapse (7). nPIST is then recycled to the Golgi (8). This Figure is reproduced in colour in Molecular Membrane Biology online.
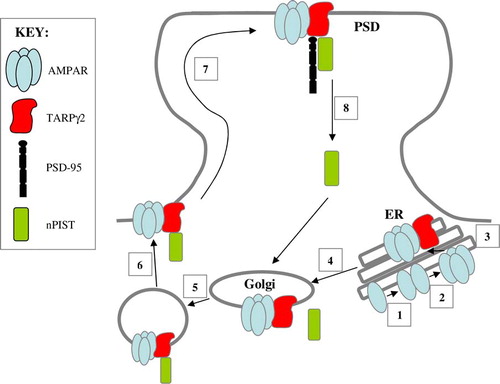
Figure 4. TARPγ2 and its associated proteins. The putative secondary structure of TARPγ2 consists of four transmembrane domains with cytosolic amino and carboxy termini. The C-terminus contains a PDZ binding motif (-RRTTPV) which interacts with MAGUKs (e.g., PSD-95 and MAGI-2) at the synapse. MAP1 LC2 and nPIST interact with TARPγ2 upstream of the PDZ binding domain. This Figure is reproduced in colour in Molecular Membrane Biology online.
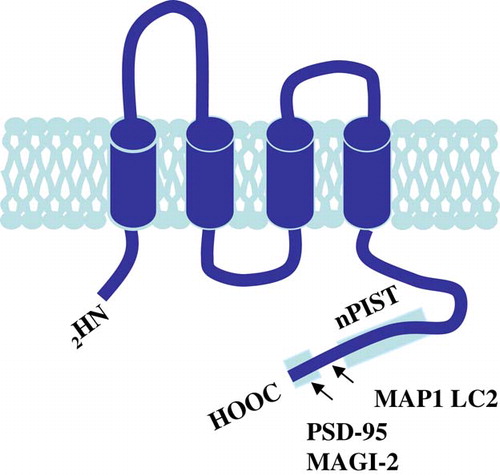
Figure 5. GABAR α6 subunit expression is downregulated in the cerebellum of the stargazer mutant mouse. The GABAR α6 subunit is downregulated in the cerebellar granule cells of the stargazer mutant mouse cerebellum, as a consequence of the stargazer mutation. (A) Immunohistochemical staining of the distribution of the GABAR α6 subunit in the cerebellum of a control (+/+:+/stg, left) and a stargazer (stg/stg, right) mouse. Note the reduction in intensity of staining, in the cerebellar granule cell layer (GL) of the stargazer mutant. ML is the molecular layer. (B) Immunoblot analysis of adult control (+/+:+/stg, left) versus stargazer (stg/stg, right) cerebellum, probed for GABAR α6 subunit expression. Arrow indicates the protein band corresponding to the GABAR α6 subunit.
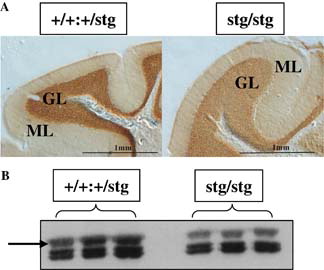
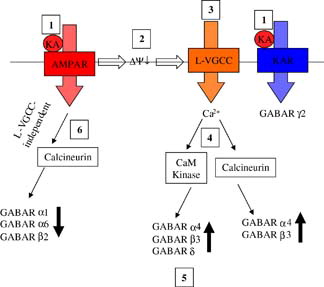
Figure 6. The effects and signalling pathways involved following activation of non-NMDA receptors on GABAR expression in mouse cerebellar granule neurons. Exogenously applied kainic acid activates both AMPAR and KAR (1). AMPAR activation evokes membrane depolarization (2) and subsequent activation of L-type voltage-gated calcium channels (3) leading to Ca2 + influx and activation of Ca2 + /calmodulin dependent protein kinase (CaM Kinase) and calcineurin (4). CaM kinase activation results in upregulation and increased membrane surface expression of GABAR α4, β3 and δ (5). Calcineurin activation by this route leads to upregulation of α4 and β3. AMPAR activation also leads to an L-type VGCC-independent activation of calcineurin resulting in downregulation of GABAR α1, α6 and β2 expression (6). KAR activation leads to downregulation of GABAR γ2 expression via a mechanism that is independent of depolarization, calcineurin, CaM kinase and NMDA receptors. Reproduced with permission from Wiley-Blackwell Publishing, Payne HL, Ives JH, Sieghart W, Thompson CL. AMPA and Kainate receptors mediate mutually exclusive effects on GABAA receptor expression in cultured mouse cerebellar granule neurons. Journal of Neurochemistry. This Figure is reproduced in colour in Molecular Membrane Biology online