Figures & data
Figure 1. Steady state anisotropy of TMA-DPH bound to outer membrane of CaCl2-treated competent cells of E. coli XL1Blue, when the cells were subjected to repetitive heat-pulse (0°C→42°C) and cold-shock (42°C→0°C) steps of the transformation process.
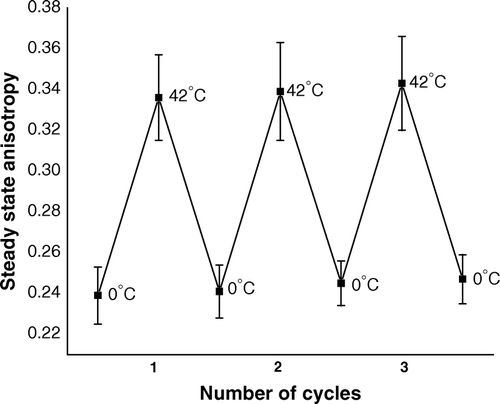
Figure 2. Pulse excitation fluorescence of TMA-DPH bound to outer membrane of E. coli XL1Blue cells after the steps of competence generation (at 0°C), heat-pulse (0°C→42°C) and cold-shock (42°C→0°C). (A) Fluorescence life-times of TMA-DPH; (B) Time-resolved anisotropies of TMA-DPH.
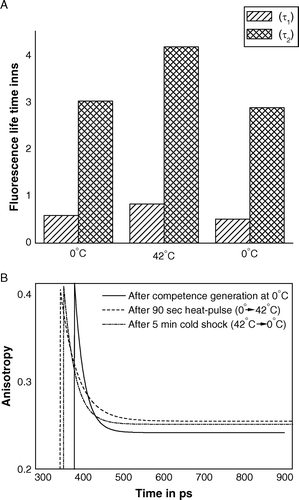
Figure 3. (A) DPH fluorescence, as a measure of lipid release from competent cell surface. (B) Result of Bradford assay, as a measure of protein release from competent cell surface, when cells were subjected to repetitive heat-pulse (0°C→42°C) and cold-shock (42°C→0°C) steps.
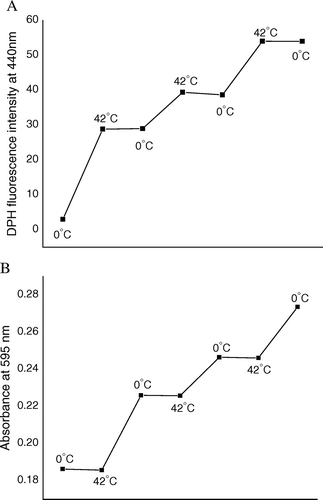
Figure 4. (A) Release of AP from the surface of E. coli XL1Blue cells after the steps of competence generation (at 0°C), heat-pulse (0°C→42°C) and cold-shock (42°C→0°C). (B) SDS-Polyacrylamide gel (12%) electrophoretic pattern of cell supernatants. Lane a: supernatant of cells suspended in 100 mM CaCl2 and kept at 0°C for 30 min; lane b: supernatant of CaCl2-treated competent cells after heat-pulse step and lane c: supernatant of competent cells after cold-shock step. (C) 2D gel electrophoresis pattern of the supernatant of competent cells after the cold-shock step.
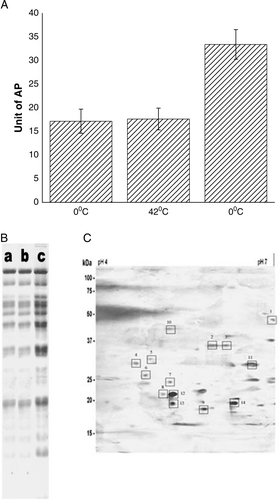
Figure 5. AFM images of the native E. coli XL1 Blue cell in phosphate buffer (images A & B), after generation of competence in 100 M CaCl2 at 0°C (images C & D), after heat pulse at 42°C for 90 s (image E) and after cold shock at 0°C for 5 min (image F). The scanning area of the images A and C of entire bacteria were 2×2 µm2. Two dimensional images B, D, E and F were acquired by zooming into the boxed area (0.7×0.7 µm2) of the images A and C. In every case 15 individual cells were studied. This Figure is reproduced in colour in Molecular Membrane Biology online.

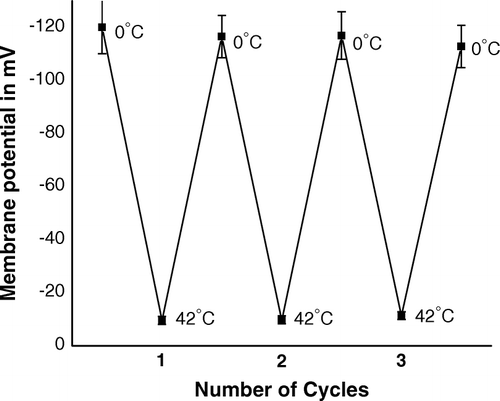
Figure 6. (TR)E of the CaCl2-treated competent cells of E. coli XL1Blue, when the cells were subjected to repetitive heat-pulse (0°C→42°C) and cold-shock (42°C→0°C) steps.
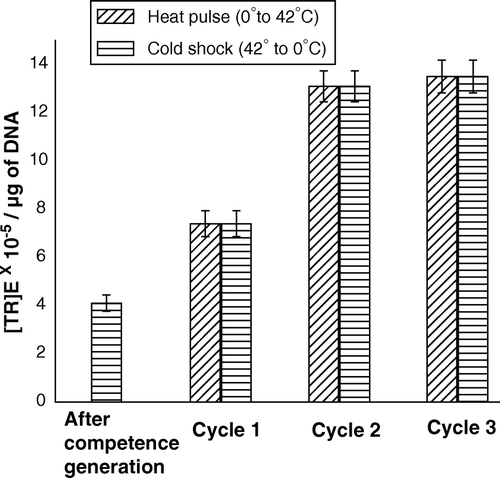
Figure 7. Steady state anisotropy of TMA-DPH bound to outer membrane of E. coli XL1Blue cells (suspended in CaCl2 of different molarities) after the steps of competence generation (at 0°C), heat-pulse (0°C→42°C) and cold-shock (42°C→0°C).
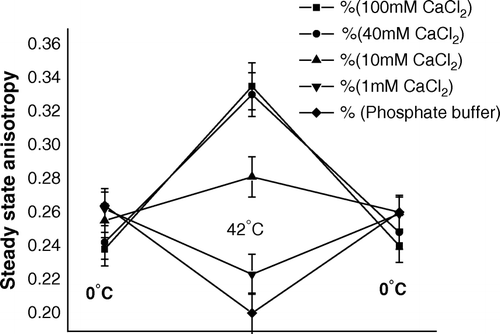
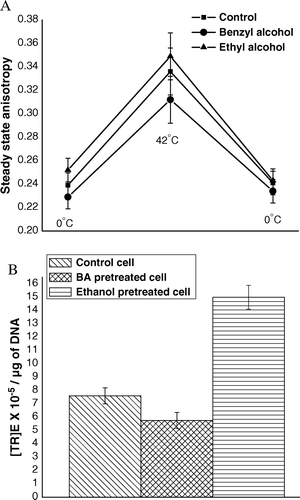
Figure 8. (A) Steady state anisotropy of TMA-DPH bound to outer membrane of E. coli XL1Blue cells (previously treated with 5% (v/v) ethanol and 30 µM benzyl alcohol separately) after the steps of competence generation (at 0°C), heat-pulse (0°C→42°C) and cold-shock (42°C→0°C). (B) (TR)E of E. coli XL1Blue cells previously treated with 5% (v/v) ethanol and 30 µM benzyl alcohol separately.