Figures & data
Figure 1. Analysis of purified CorA ion channels from M. Jannaschii (MJ1033) and P. Aeruginosa (PA5268) by PFO-PAGE and size exclusion chromatography. (A) Silver stained 7.5% PFO-PAGE gel of purified MJ1033. Whereas DDM-purified MJ1033 shows a predominant band assignable to a tetramer (160 kDa) or pentamer (200 kDa), LDAO-purified MJ1033 appears predominantly monomeric; additional weaker bands may represent a dimer, a tetramer and a pentamer. (B) Superdex 200 size exclusion chromatography of MJ1033 purified in LDAO (light grey dotted line) and DDM (blue solid line) and PA5268 purified in LDAO (black triangles). The position of the void volume of the column (600 kDa) and the elution volume of a 150 kDa marker protein are shown. Consistent with the PFO-PAGE, the major peak at 11 ml in DDM-purified MJ1033 has a retention volume consistent with a tetramer or pentamer, whereas the LDAO-purified MJ1033 has a major peak at 13 ml eluting after the 150 kDa marker; the smaller peak at 11 ml may represent the fainter putative tetramer and pentamer PFO-PAGE bands. (C) Anti-T7 Immunoblot of an 11% PFO-PAGE gel of purified PA5268 and MJ1033, with the position of bands in a prestained molecular weight ladder indicated. MJ1033, but not PA5268, reveals a non-covalent oligomer as a high molecular weight band removed by SDS treatment (marked with an asterisk). Faint high molecular weight bands are observed in the immunoblot of LDAO-purified PA5268; these presumably represent an impurity rather than a non-covalent oligomer as they are observed both with and without SDS-treatment of the sample. This Figure appears in colour in the online version of Molecular Membrane Biology.
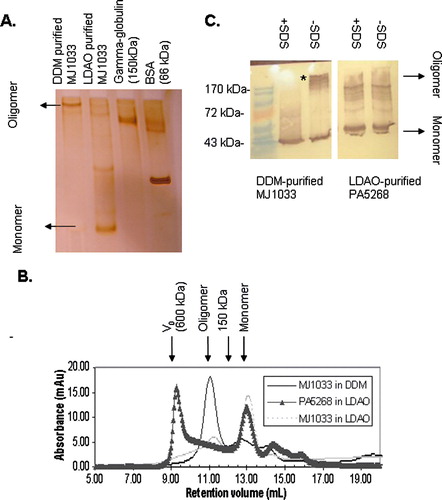
Figure 2. Selection of detergent for purification and crystallization of ABC transporter ModBC. (A) Anti-HSV immunoblot of a PFO-PAGE analysis of detergent extracts from crude Escherichia coli membranes containing overexpressed ModBC. A band corresponding to the intact ModBC complex is only apparent in Triton X-100, C12E8, Zwittergent-3,12 and Lyso FOS-CHOLINE-12. In some cases there are two oligomeric species identifiable, the faster running band having a reasonable mobility to represent the fully assembled transporter (130 kDa). The upper species (marked with an asterisk) may represent a dimer of fully assembled transporters; the capacity of fully assembled ABC transporters to form strong dimers has been reported elsewhere Citation[15], Citation[16]. (B) Superdex 200 analytical size exclusion chromatogram of ModBC after Ni-NTA purification in C12E8 and DDM. SDS-PAGE shows ModB and ModC subunits co-eluting in the peak in the highlighted portion of the C12E8 SEC chromatogram. In DDM the subunits elute separately in the asterisk-highlighted peaks at 7 ml (ModC) and 13 ml (ModB). This Figure appears in colour in the online version of Molecular Membrane Biology.
![Figure 2. Selection of detergent for purification and crystallization of ABC transporter ModBC. (A) Anti-HSV immunoblot of a PFO-PAGE analysis of detergent extracts from crude Escherichia coli membranes containing overexpressed ModBC. A band corresponding to the intact ModBC complex is only apparent in Triton X-100, C12E8, Zwittergent-3,12 and Lyso FOS-CHOLINE-12. In some cases there are two oligomeric species identifiable, the faster running band having a reasonable mobility to represent the fully assembled transporter (130 kDa). The upper species (marked with an asterisk) may represent a dimer of fully assembled transporters; the capacity of fully assembled ABC transporters to form strong dimers has been reported elsewhere Citation[15], Citation[16]. (B) Superdex 200 analytical size exclusion chromatogram of ModBC after Ni-NTA purification in C12E8 and DDM. SDS-PAGE shows ModB and ModC subunits co-eluting in the peak in the highlighted portion of the C12E8 SEC chromatogram. In DDM the subunits elute separately in the asterisk-highlighted peaks at 7 ml (ModC) and 13 ml (ModB). This Figure appears in colour in the online version of Molecular Membrane Biology.](/cms/asset/34c4ed96-3b6e-4bce-a737-9fc024eaa858/imbc_a_345021_f0002_b.jpg)