Figures & data
Table I. Data collection and refinement statistics.
Figure 1. Comparison of growth of an E. coli wild-type strain with an acrB deletion strain in the presence of deoxycholate (DOC). thin line, BL21(DE3) in absence of bile acid; thin dashed line, BL21(DE3) with 5 mM addition of deoxycholate; thick line, BL21(DE3)ΔacrB::KmR in absence of bile acid, thick dashed-line, BL21(DE3)ΔacrB::KmR with 5 mM addition of deoxycholate.
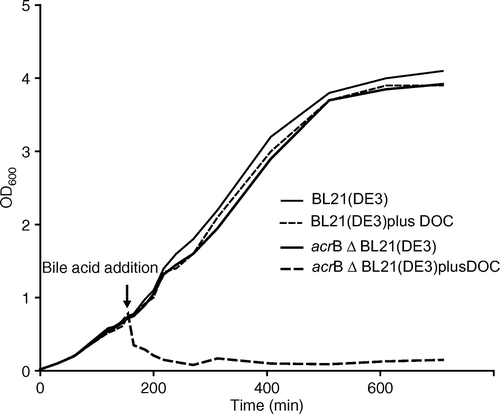
Figure 2. Fractions of a standard membrane protein-GFP-His8 purification were loaded onto a 12% SDS-polyacrylamide gel and then stained with Coomassie to illustrate isolation of native AcrB. (1) MP-GFP-His8 elution from first IMAC; (2) overnight His6-TEV protease digested material; (3) flow-through of second IMAC step; and (4) elution of bound material from second IMAC (bands corresponding to AcrB, MP-GFP-His8, His6-TEVp, and MP are as indicted on gel).
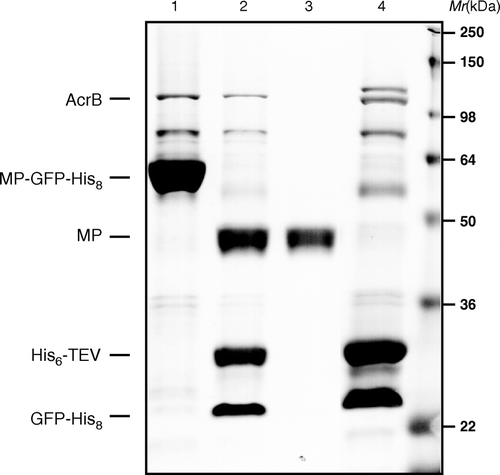
Figure 3. Overall structure of AcrB trimer. The bound deoxycholate in the periplasmic vestibule is shown in yellow stick. Boxed area is a close-up view of the vestibule site with the deoxycholate and side chains that are involved in the binding shown in grey stick.
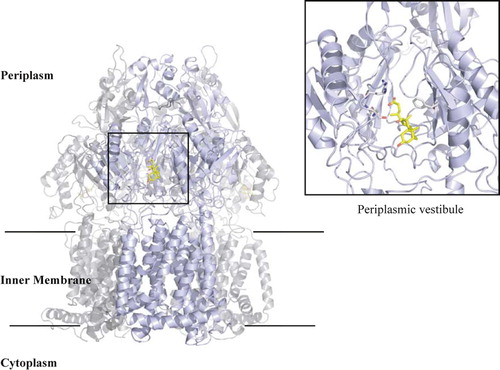
Figure 4. Binding of deoxycholate in the periplasmic vestibule of AcrB. (a) Electron density for native AcrB (purple), (b) deoxycholate bound prior to adding the ligand (light blue), (c) deoxycholate density after including it in the refinement (dark blue). The 2Fo-Fc maps are contoured at 1.0σ and deoxycholate (pink, contoured at 1.0σ) are shown for clarity, calculated after some model building (prior to deoxycholate being built). Some of the vestibule amino acid side chains are shown. Deoxycholate (yellow sticks) is included for reference. Nitrogen atoms are shown in blue and oxygen in red.
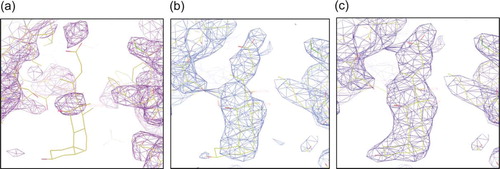
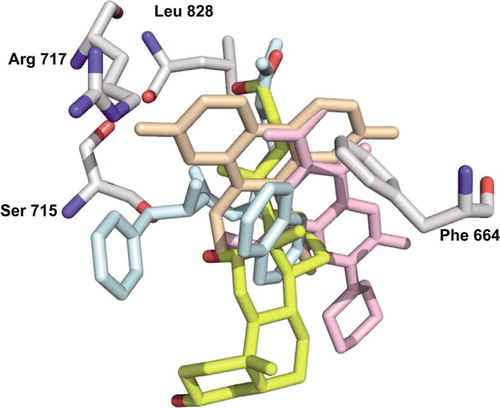
Figure 5. Alignment of deoxycholate (yellow) with ethidium (light orange), Phe-Arg-β-naphthylamide (light blue), and ciprofloxacin (pink). The residues involved in the binding of deoxycholate are shown as grey sticks. Arg717 is only shown as a reference since there is no direct contact with the deoxycholate.