Figures & data
Figure 1. The ‘classic’ yeast 2-hybrid system. (A) A yeast S. cerevisiae transcriptional activator consisting of both AD (activating domain) and BD (binding domain) can induce reporter gene transcription. (B) A hybrid of protein X fused to BD (BD-X) alone is incapable of inducing gene transcription. (C) A hybrid of protein Y fused to AD (Y-AD) alone is incapable of inducing gene transcription. (D) Interaction of protein X-BD with Y-AD brings the AD into close proximity to the promoter of the gene, thus recruiting RNA polymerase II to transcribe the reporter gene. This Figure is reproduced in colour in Molecular Membrane Biology online.
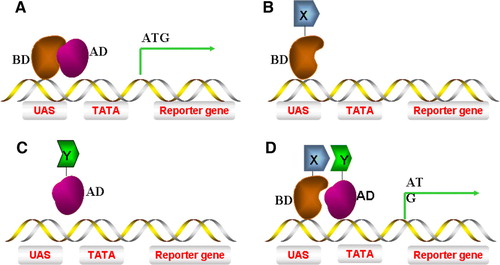
Figure 2. The Sos Y2H system. (A) Grb2 binds to a phosphotyrosine epitope in the cytoplasmic domain of an activated growth factor receptor and mediates recruitment of Sos (Cdc25), which in turn leads to Ras activation. (B) A temperature-sensitive mutation in the Ras guanyl nucleotide exchange factor Cdc25–2, blocks yeast growth at 36°C. A cytosolic protein domain Y is fused to Sos that allows interaction with an integral membrane protein X, leading to Ras activation and cell growth at 36°C. This Figure is reproduced in colour in Molecular Membrane Biology online.
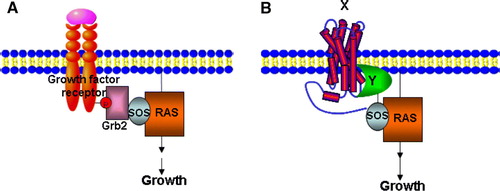
Figure 3. A G-protein coupled receptor-based Y2H system. (A) The activation of a G-protein coupled receptor can lead to dissociation of Gβγ from Gα and then to the activation of downstream effectors. (B) In the Gγ-deficient yeast mutant stain, a hybrid cytosolic protein Y interacts with Gβγ and membrane-bound protein X simultaneously and prevents interaction of Gβγ to activated Gα, thus inhibiting downstream signalling and gene expression. This Figure is reproduced in colour in Molecular Membrane Biology online.
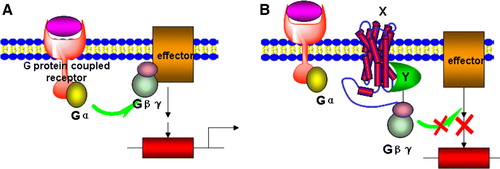
Figure 4. The split-ubiquitin Y2H system. (A) Native ubiquitin can be divided into two parts: Cub and Nub. Fusion of Cub to the transcription activator LexA-VP16 followed by co-expressed with Nub, leads to spontaneous assembly of a native ubiquitin molecule and recognition by a UBP, cleavage and liberation of LexA-VP16. (B) Introduction of the I13G mutation into Nub (Nub-G) causes reduced affinity for Cub, lack of ubiquitin assembly and no cleavage. (C) The interaction of integral membrane protein X with either the cytosolic protein Y or an integral membrane protein Z can bring NubG and Cub into close proximity, followed by UBP-mediated cleavage which releases the LexA-VP16 transcription factor, allowing it to enter into the nucleus and stimulate reporter gene transcription.
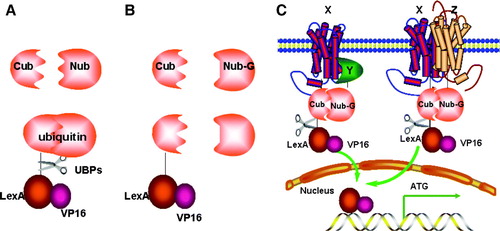
Figure 5. Yeast three-hybrid (Y3H) systems. (A) Interaction between protein X and protein Y must be mediated by protein Z and assembly of this protein complex stimulates reporter gene transcription. (B) Protein X can only interact with protein Y (and stimulate gene transcription) after being modified by protein Z, e.g. phosphorylation. This Figure is reproduced in colour in Molecular Membrane Biology online.
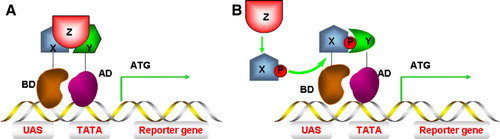
Figure 6. Y3H screen for compounds that inhibit protein-protein interactions. (A) The association of protein X and protein Y induces transcription of URA3, leading to URA3p production and conversion of 5-FOA into a toxic metabolite and cell death. (B) A small chemical compound c can inhibit the interaction between protein X and protein Y, protecting against cell death causing cell survival instead. This Figure is reproduced in colour in Molecular Membrane Biology online.
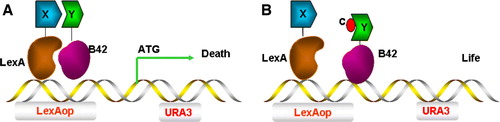
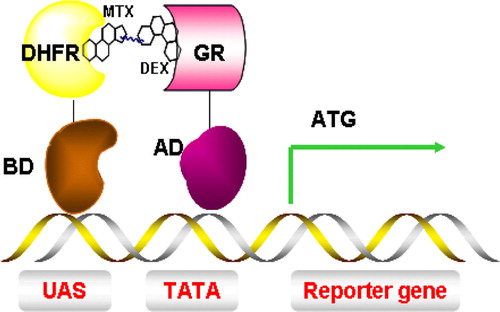
Figure 7. Y3H system for the detection of small molecules and protein interactions. Interaction between dihydrofolate reductase (DHFR) and glucocorticoid receptor (GR) is mediated by a dexamethasone-methotrexate (Mtx-Dex) heterodimer, thus inducing transcription of a reporter gene. This Figure is reproduced in colour in Molecular Membrane Biology online.