Figures & data
Box 1. Chemical structure of the spin labelled analogues of sphingolipids with a short sn-2 chain: Spin-labelled ceramide (SL-Cer); Spin-labelled Galactosylceramide (SL-GalCer); Spin-labelled Glucosylceramide (SL-GlcCer); Spin-labelled Lactosylceramide (SL-LacCer); Spin-labelled Sphingomyelin (SL-SM); Spin-labelled dihydroceramide (SL-dihyCer).
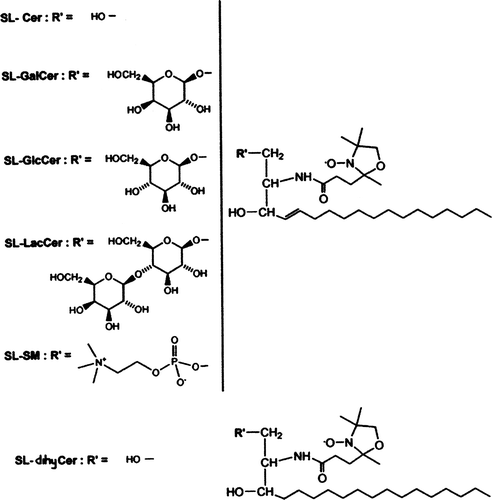
Figure 1. ESR spectra for different spin labelled phosphatidylcholines embedded in a ld membrane (dashed line) or in lo membrane (solid line). (A) SL-PC (10,3) (the probe is attached near the polar head group). (B) SL-PC (5,10) (the probe is attached at carbon 11 of the sn2 chain) and (C) SL-PC (1,14) (the probe is attached near the methyl terminal of the alkyl chains at carbon 15). The line broadening, indicative of a decrease on molecular motion, was systematically observed for all probes when inserted in more ordered phases. Differences in spectra line shape depends on the position of the nitroxide group on the acyl chain.
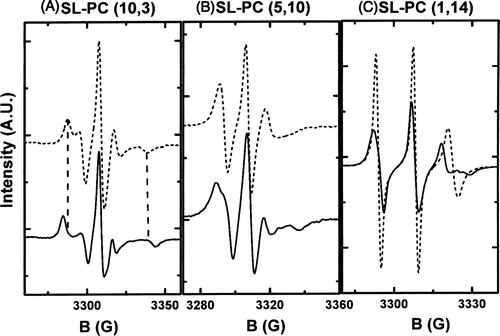
Figure 2. (A) Inward movement of SL-GlcCer (solid symbols) and SL-GalCer (open symbols) in egg-PC LUVs containing 0% (squares), 25% (circles) or 44% (triangles) cholesterol at 20°C. (B) Inward movement of SL-GalCer in (POPC/SM/Chol; 20/20/60 mol) LUVs (lo phase, open rhombus) at 20°C. For comparison, data from A corresponding to the inward transport of SL-GalCer in more fluid phases, were also replotted (squares and triangles). After 10 min incubation of SL-lipids with LUVs on ice, tubes were transferred to 20°C. At indicated time points, samples were mixed with ice-cold BSA (final concentration 5%) and incubated on ice for 30 sec before addition of ascorbic acid (final concentration 10 mM). SL-lipid inaccessible to reduction was quantified by ESR. Results are means +/− SEM of at least n=3 independent experiments.
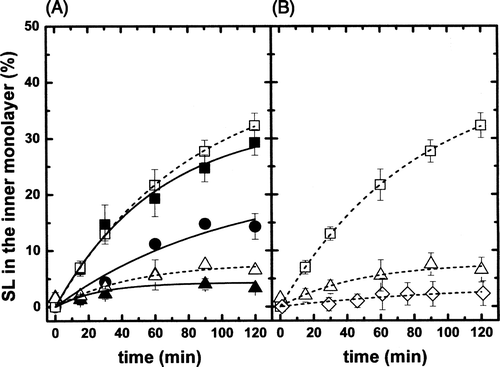
Figure 3. (A) Inward movement of SL-SM in POPC LUVs (ld phase, solid squares) and in (POPC/SM/Chol; 20/20/60 mol) LUVs (lo phase, open squares). Temperature was 20°C. (B) Inward movement of SL-LacCer in POPC LUVs (ld phase, solid squares) and in (POPC/SM/Chol; 20/20/60 mol) LUVs (lo phase, open squares). Temperature: 20°C. Results are means +/- SEM of two duplicated independent experiments.
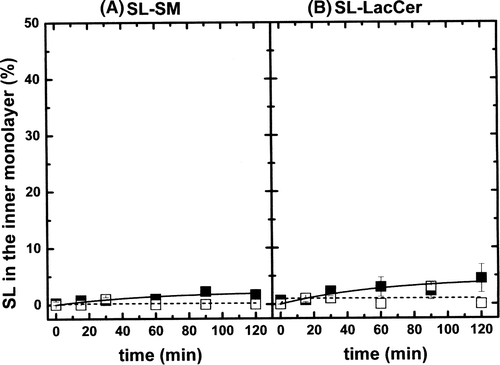
Figure 4. (A) Inward movement of SL-GlcCer (solid symbols) and SL-GalCer (open symbols) in egg-PC LUVs containing 0% (squares) or 44% (triangles) cholesterol at 37°C. For comparison, data from A corresponding to the inward teransport of SL-GlcCer at 20°C were also replotted (dashed-dot lines). (B) Inward movement of SL-LacCer in POPC LUVs (ld phase, solid squares) and in (POPC/SM/Chol; 20/20/60 mol) LUVs (lo phase, open squares) at 37°C.
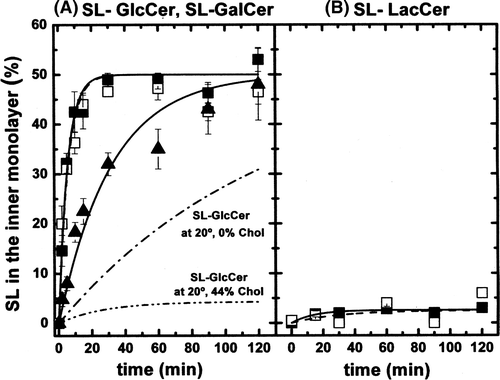
Figure 5. (A) Outward movement of SL-Cer in POPC LUVs (ld phase, solid squares) and in(POPC/SM/Chol; 20/20/60 mol) LUVs (lo phase, open squares). Temperature: 20°C. (B) Outward movement of SL-dihyCer in POPC LUVs (ld phase, solid squares) and in (POPC/SM/Chol; 20/20/60 mol) LUVs (lo phase, open squares). Temperature: 20°C.
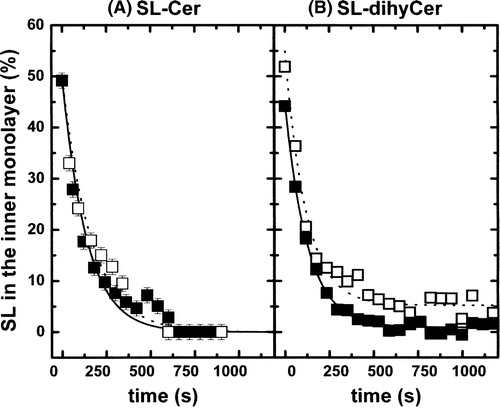
Table I. Halftimes τ1/2 (in minutes) of flip-flop for sphingolipids analogues in different lipid phases at 20 and 37°C.