Figures & data
Figure 1. Hydrophobic moment, hydrophobicity, interfaciality distribution, and relative position of the SARSL peptide used in this study along the SARS-CoV spike S2 domain, assuming it forms an α-helical wheel Citation[16]. Only positive bilayer-to-water transfer free-energy values are depicted (the darker, the higher). The scheme of the structure of SARS-CoV spike glycoprotein S2 (amino acid residues 758–761 to 1255 for the full-length) according to literature consensus is also shown. The relevant functional regions are highlighted: the putative fusion peptide (FP), the internal fusion peptide (IFP), the predicted heptad repeat regions pertaining HR1, HR2, and the pre-transmembrane (PTM), transmembrane (TM) domains Citation[14], Citation[16], Citation[19], Citation[20], Citation[34]. The sequence of the peptide used in this work is also shown.
![Figure 1. Hydrophobic moment, hydrophobicity, interfaciality distribution, and relative position of the SARSL peptide used in this study along the SARS-CoV spike S2 domain, assuming it forms an α-helical wheel Citation[16]. Only positive bilayer-to-water transfer free-energy values are depicted (the darker, the higher). The scheme of the structure of SARS-CoV spike glycoprotein S2 (amino acid residues 758–761 to 1255 for the full-length) according to literature consensus is also shown. The relevant functional regions are highlighted: the putative fusion peptide (FP), the internal fusion peptide (IFP), the predicted heptad repeat regions pertaining HR1, HR2, and the pre-transmembrane (PTM), transmembrane (TM) domains Citation[14], Citation[16], Citation[19], Citation[20], Citation[34]. The sequence of the peptide used in this work is also shown.](/cms/asset/98c28d25-da45-4207-8442-aa5619cac96d/imbc_a_392792_f0001_b.gif)
Figure 2. (A) Change on the Trp fluorescence intensity of the SARSL peptide in the presence of increasing lipid concentration, (B) Stern-Volmer plots of the quenching of the Trp fluorescence emission of SARSL by acrylamide in aqueous buffer (∇), and in the presence of LUVs, (C) Doxyl NS quenching (5NS, open symbols, and 16NS, black symbols) of the Trp fluorescence of the SARSL peptide in the presence of LUVs at different lipid compositions. The lipid compositions used were EPC (▪), BPS (▴), EPG (•), EPA (♦). The spin label concentration is the effective quencher concentration calculated as stated in the Material and Methods section.
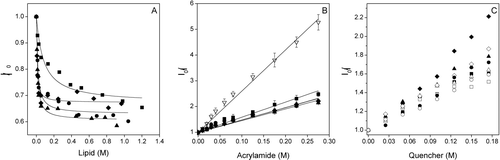
Table I. Partition coefficient (Kp), spectral shift (Δλ), anisotropy value, acrylamide Stern-Volmer (KSV) quenching constant obtained from the fluorescence of the Trp residue of the SARSL peptide in buffer, in the presence of different model membranes.
Table II. Fluorescence lifetime components τi, normalized amplitudes αι, and fluorescence mean lifetime (amplitude weighted, , and intensity weighted, <τ > ) of the Trp residue of the SARSL peptide in buffer, in the presence of phospholipid model membranes at 25°C. The reduced χ2 values of the fits to the experimental fluorescence decays are also given. The peptide/lipid ratio was 1:30.
Table III. Long rotational correlation time φ from the anisotropy decay of the peptide SARSL in buffer solution at different temperatures. The global chi-square is also given. For comparison, the rotational correlation time for different aggregates of the SARSL peptide at those temperatures is also indicated. The inclusion of a very short rotational correlation time (<100 ps) was necessary to account for the initial depolarization, but its value could not be accurately determined.
Table IV. Fitting parameters for the anisotropy decay of the peptide SARSL (rotational correlation time φ, associated depolarization β, and limiting anisotropy r∞) in lipid membranes of several compositions at 25°C. The global chi-square is also given.
Figure 3. (A) Effect of the SARSL peptide on membrane rupture of LUVs at different lipid compositions, at different lipid-to-peptide molar ratios. The lipid compositions used were EPC (▪), EPC/BPS/CHOL at a molar ratio of 5:3:1 (▴), EPG (•), EPC/EPG/CHOL at a molar ratio of 5:3:1 (▾), EPC/EPA/Chol at a molar ratio of 5:3:1 (♦). (B) Insertion of the SARSL peptide into lipid monolayers. Increments in surface pressure (Δπ) of lipid monolayers due to the addition of SARSL peptide into the subphase is illustrated as a function of the initial pressure (π0). The lipid compositions used were POPC (•), POPS (▴), POPG (▪).
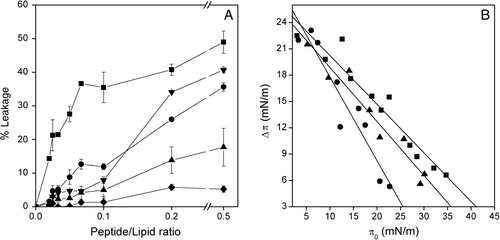
Figure 4. Infrared spectra in the Amide I’ region of the SARSL peptide in solution (A), and in the presence of liposomes composed of (B) EPC, (C) EPG at a phospholipid/peptide molar ratio of 15:1 in D2O buffer at 25°C.
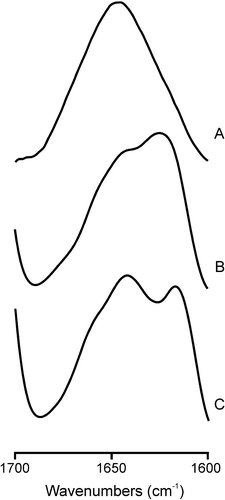
Table V. Fluorescence lifetime components τι, normalized amplitudes αι, and fluorescence mean lifetimes (amplitude weighted, , and intensity weighted, <τ > ) of DPH in phospholipid model membranes in the absence, in the presence of the SARSL peptide below, above the phospholipid Tm. * From ref. Citation[34].