Figures & data
Figure 1. Regulation of excitatory synapses by Palmitoylated Fyn. Left panel: Several key pre- and post-synaptic proteins are palmitoylated (marked with yellow lipid). These palmitoyl-proteins include the tyrosine kinase Fyn, which is likely localized to the postsynaptic plasma membrane by dual lipid modification (myristate plus palmitate). Under normal conditions, Fyn phosphorylates NMDA receptors and the PAT DHHC5, preventing their internalization. Right panel: Elevated synaptic activity leads to dephosphorylation of DHHC5 at the Fyn site, triggering DHHC5 internalization to recycling endosomes (RE). Here, DHHC5 palmitoylates delta-catenin and the two proteins are trafficked back to the spine membrane, where delta-catenin associates with N-cadherin to increase the synaptic pool of AMPA-type glutamate receptors. Note that several other synaptic palmitoyl-proteins, such as PSD-95, are omitted from the schematic for clarity. Also not pictured is the synaptodendritic PAT DHHC2, which palmitoylates several dendritic/postsynaptic proteins, and may also palmitoylate Fyn.
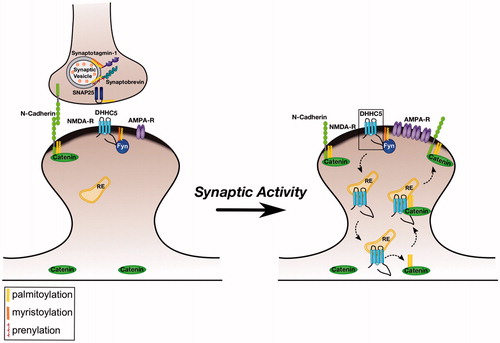
Figure 2. Control of spine morphology by palmitoylated Cdc42 and LIMK1. Left panel: Palmitoyl-LIMK1 on the dendritic spine membrane is phosphorylated and activated via a pathway that involves membrane-bound upstream activators such as Rac, Cdc42 (which is also palmitoylated) and PAK. In contrast, any non-palmitoylated LIMK1 in the spine ‘core’ remains inactive. This palmitoylation-dependent control of LIMK1 localization and activity may facilitate juxtamembrane phosphorylation and inactivation of cofilin, thus allowing spine-specific, and perhaps even sub-spine, control of actin dynamics. Right panel: Elevated synaptic activity triggers Rac/Cdc42/PAK activation and recruitment of additional cofilin to spines (Bosch et al., Citation2014; Murakoshi et al., Citation2011). Both these events are required for activity-dependent spine enlargement, which also depends on palmitoyl-LIMK1 (George et al., Citation2015). Control of sub-spine actin polymerization by this signaling pathway may provide the force required for the enlargement of specific spines that is frequently observed during LTP.
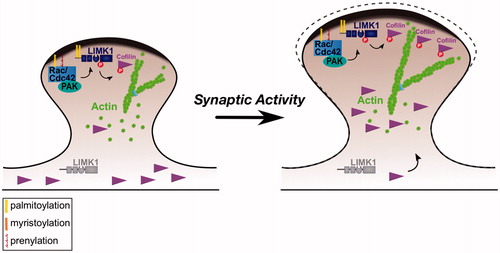
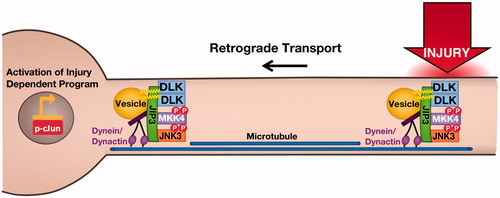
Figure 3. Axonal retograde signaling by palmitoylated DLK-JNK pathway kinases. Palmitoylation is essential for DLK’s ability to convey retrograde injury signals following distal axonal injury. At the cellular level, palmitoylation targets DLK to trafficking vesicles where DLK forms complexes with its direct substrate MKK4. These complexes also contain the scaffold protein JIP3, which binds dynein/dynactin retrograde motor proteins, and may also involve the downstream palmitoyl-kinase JNK3, which is heavily implicated in axonal retrograde signaling. In addition to this role in DLK localization, palmitoylation is necessary for DLK’s ability to activate the JNK pathway. This additional ‘security feature’ ensures that depalmitoylated DLK does not inappropriately phosphorylate cytosolic MKKs.