Figures & data
Figure 1. Apoptosis versus necrosis. Apoptosis (top) is an active and inherently controlled means of cell death. The physiological features of programed cell death include: cell shrinking, chromatin condensation, membrane blebbing and pinching off into apoptotic bodies that can be phagocytized by macrophages. Necrosis (bottom) is invariably caused by noxious stimuli as a result of an irreversible disturbance of cellular homeostatic mechanisms. Not involved in the control of cell populations, this form of cell death differs in that in comprises of cell swelling, loss of membrane integrity and organelles, degradation of DNA and eventual cell lysis into the extracellular environment.
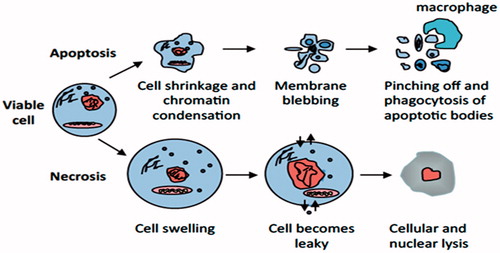
Figure 2. Intrinsic pathway of apoptosis. The intrinsic pathway shows an induction or post-translational activation of BH3-only protein upon induced stress, which results in the inactivation of some BCL-2 family members. This relieves inhibition of BAX and BAK activation, which in turn promotes apoptosis. BAX and/or BAK will be activated by BH3-only proteins. The pro-apoptotic Bcl-2 family members Bax and Bak oligomerize and undergo conformational changes in the mitochondrial membrane of stressed cells forming channels that permit the release of pro-apoptotic factor, cytochrome c, from mitochondria. Once released, activation of APAF1 into an apoptosome takes place followed by caspases-9 and-3 activation that ultimately causes the proteolytic dismantling of the dying cell.
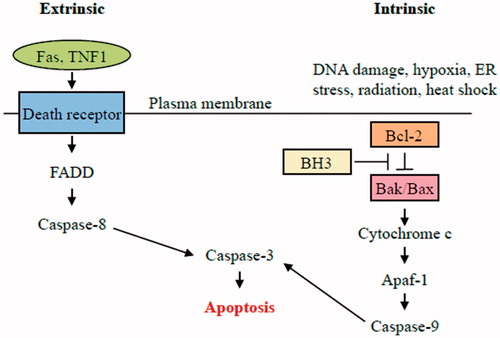
Figure 3. Structure of the Bcl-2 protein family members. The BH1-3 domain forms a hydrophobic receptor domain able to sequester the BH3 domain-only proteins. BH1/2/3 domain of pro-survival Bcl-2 proteins acts as a receptor for the BH3 domain of other Bcl-2 family members.
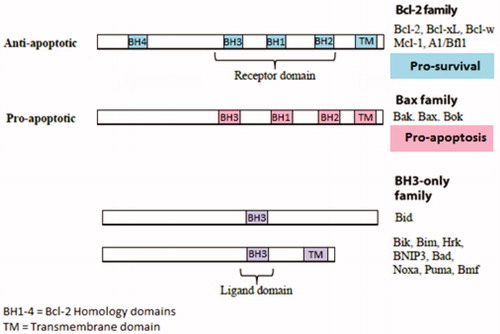
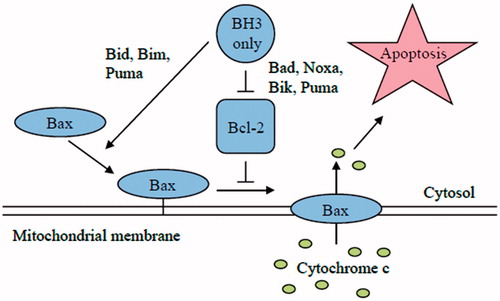
Figure 4. Schematic model of apoptosis regulation by Bcl-2 members protein family. Apoptosis is initiated by the oligomerization of Bax (or Bak) in the mitochondrial outer membrane, which causes the formation of a channel for the release of cytochrome c and other apoptogenic factors. The anti-apoptotic Bcl-2 family members inhibit Bax and Bak oligomerization. The pro-apoptotic BH3-only members of the Bcl-2 family are the direct sensors of cellular stress. They trigger cell death by either inhibiting the anti-apoptotic Bcl-2 members or in the case of BIM and tBID by directly activating BAX and BAK. The BH3-only proteins are regulated by a number of mechanism included increased gene expression or posttranslational modifications.
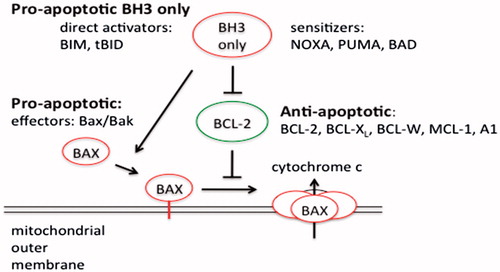
Figure 5. Schematic model showing the binding specificity of different BH3-only proteins. Bim, Puma and tBid (promiscuous members) are able to bind to all anti-apoptotic Bcl-2 family members. On the other hand, Bad and Noxa (selective members) bind only to certain anti-apoptotic Bcl-2 family members.
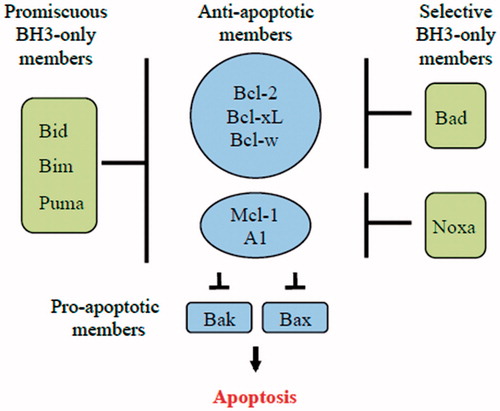
Figure 6. Comparison of the direct and indirect model of Bax and Bak activation. In the direct model, the BH3-only members (Bim, tBid and PUMA) act as activators and bind to Bax and Bak directly to induce pore formation in the OMM that permits the release of cytochrome c. On the other hand, the remaining BH3-only proteins act as sensitizers and bind to the Bcl-2 members, releasing bound Bim and tBid and allowing them to directly activate Bak and Bax. The indirect model shows that BH3-only proteins do not bind directly to Bax and Bak; however, they engage the anti-apoptotic proteins, causing the release of Bak and Bax. Some BH3-only proteins bind to specific anti-apoptotic Bcl-2 family proteins (selective) while others bind to all anti-apoptotic Bcl-2 proteins (promiscuous).