Figures & data
Figure 1. (a) Cartoon of Ptr2p Topology. Filled circles represent amino acids 1–601 of native Ptr2p; open circles denote the placement of the C-terminal FLAG and His epitope tags. TM5 is indicated by the oval. (b) Enlarged representation of TM5 indicated by the oval in . The invariably conserved FYING residues are indicated in black. Highly, but not invariably, conserved residues are shaded grey. (c) Helical wheel representation of TM5. FYING residues are in black, and are all localized to the same half of the helix as indicated by the dotted line.
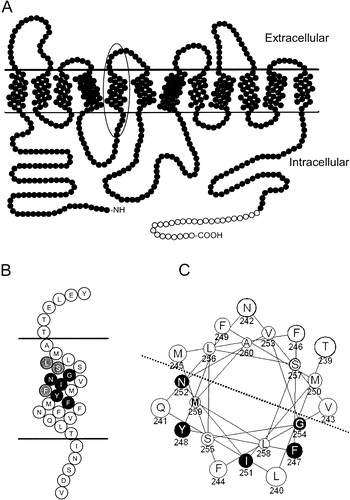
Figure 2. The FYING motif is invariably conserved in Ptr2p. The fifth transmembrane domain (TM5) of representative eukaryotic Ptr2ps for which peptide transport has been functionally verified were aligned using Clustal W. The FYING motif residues are highlighted. The superscript number before the first amino acid in each TM5 indicates the residue number of that particular protein. Names for genes encoding the transporters are listed in parenthesis.

Figure 3. Expression of wild-type and TM5 Ala-scanned mutant Ptr2p transporters in the membrane of Saccharomyces cerevisiae. Membranes were prepared from cells expressing TM5 Ala-scanned mutants (T239–A260). As controls membranes were also prepared from cells expressing wild-type (WT +) or no (WT −) Ptr2p. Membrane proteins (10 µg) were resolved by SDS-PAGE, blotted and immunodetected with anti-FLAG antibodies. Ptr2p was present in all lanes except the negative control (WT −) and the Y248A mutant. FYING mutants are indicated by dots over the corresponding residues.
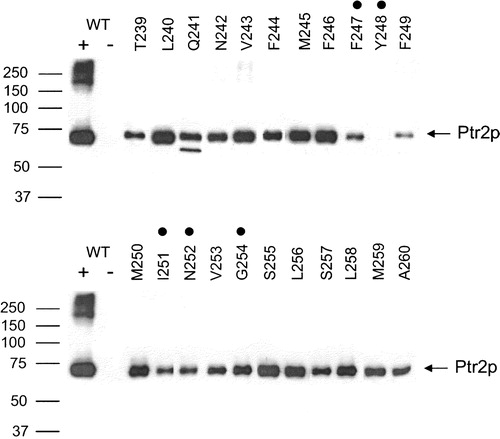
Figure 4. Growth assay for wild-type and TM5 mutant Ptr2p transporters. Cells expressing (WT) or lacking (Vector) Ptr2p or expressing the various Ala-scanned mutants were assayed for growth on medium containing the dipeptide Leu-Leu as the sole source of leucine. Growth was scored by visual comparison of spot densities as wild-type growth (+ + ), reduced growth (+) or no growth (−) as indicated below each label. FYING residues are indicated in italics. Growth on other dipeptide substrates was determined in like manner and data are presented in .
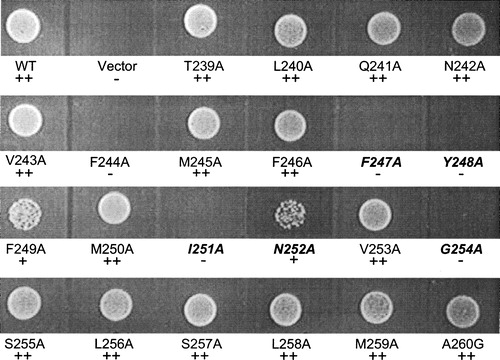
Table I. Ala-scanned mutants of TM5 which exhibit substrate preference. Yeast were plated onto media as described in experimental procedures. They were visually scored for growth by comparing spot densities (see ) to wild-type: ++, wild-type growth; +, reduced growth; −, no growth. Wild-type, positive control plasmid encoding wild-type PTR2. Vector, negative control plasmid without insert.
Figure 5. Transport of radiolabeled Leu-Leu and Met-Met-Met by wild-type and TM5 mutant transporters. Cells expressing wild-type or Ala-scanned mutant (T239–A260) Ptr2p constructs were grown under inducing conditions as described in Experimental procedures. Each point was determined in triplicate and results reported as the mean of the replicate values plus or minus standard deviation. In some cases error bars are present but not visible. Cells lacking Ptr2p (Vector) were used as a negative control. (a) The 10 min. accumulation of 14C-Met-Met-Met (○) or 3H-Leu-Leu (•) was determined. (b) Ratio of Met-Met-Met/Leu-Leu accumulation for each mutant. Background (radioactivity associated with vector control) was subtracted from each mutant prior to calculating ratios. For mutants exhibiting zero transport of both substrates, no bar was plotted. Asterisks indicate that values are significantly different (p<0.01) from wild-type.
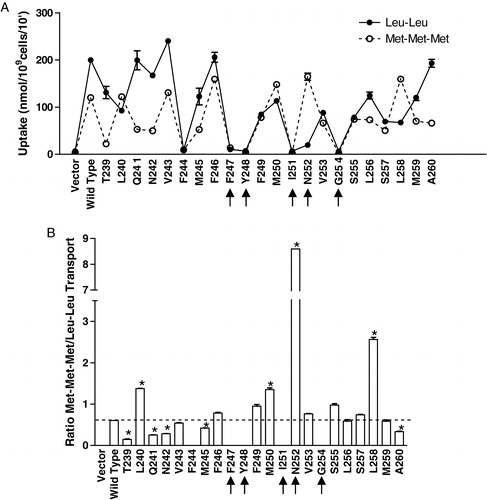
Figure 6. Effect of 10-fold excess unlabeled Leu-Leu or Met-Met-Met on 14C-Met-Met-Met transport in cells expressing wild-type or TM5 mutant transporters. Cells expressing the wild- type or mutant (F247A, M250A, L258A) Ptr2p constructs were grown under inducing conditions as described in Experimental procedures. Each point was determined in triplicate and results reported as the mean of the replicate values plus or minus standard deviation. In some cases error bars are present but not visible. Cells lacking Ptr2p (Vector) were used as a negative control. (a) The 10 min. accumulation of 14C-Met-Met-Met (160 µM) in the presence (open boxes) or absence (dark boxes) of 1.6 mM Leu-Leu. (b) The 10 min accumulation of 14C-Met-Met-Met (160 µM) in the presence (open boxes) or absence (dark boxes) of 1.6 mM Met-Met-Met.
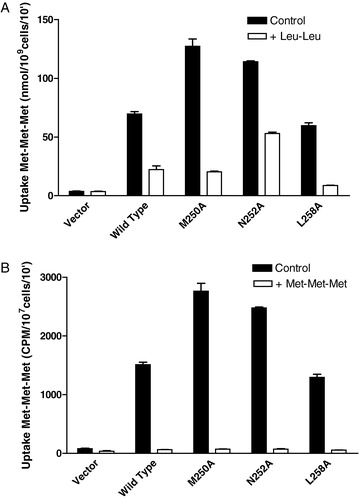
Table II. Sensitivity to toxic dipeptides. Toxic peptide (0.1 µmols) or the corresponding toxic amino acid analogue (0.1 µmols) was applied to a sterile disk and placed on a top agar lawn of cells as described in experimental procedures. The diameter (mm) of the zone of inhibition was measured. Vector, negative control plasmid without insert. Wild-type, positive control plasmid encoding wild-type PTR2.