Figures & data
Figure 1. Syntaxin 10 co-localizes with syntaxin 6 and 16 at the TGN. Cells were fixed with 4% paraformaldehyde and incubated with primary antibody against syntaxin 10 (rabbit), mouse monoclonal antibody against syntaxin 6, or mouse anti-syntaxin 16 antisera, followed by Texas-red or FITC-labeled secondary antibodies. Bar = 10 µm.
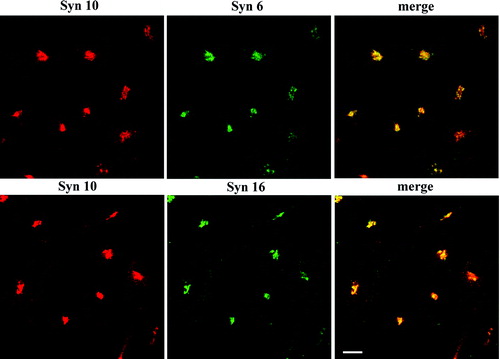
Figure 2. Syntaxin 10 interacts in vivo with TGN SNAREs: (a). HeLa cell lysates were subjected to immunoprecipitation with syntaxin 10 antibody. Immunoprecipitates were eluted, resolved on 12% SDS-PAGE gels, and subjected to Western immunoblot analyses with antibodies against syntaxin 6, syntaxin 16, Vti1-rp2 and syntaxin 2. For co-precipitation analysis with VAMP4, a construct of N-terminal myc-tagged VAMP4 is transiently transfected into 293T cells and subjected to immunoprecipitations as above and western immunoblot analysis with an anti-myc monoclonal antibody (9E10); (b). Reciprocal immunoprecipitation analyses. HeLa cell lysates were subjected to immunoprecipitation with syntaxin 6, syntaxin 16 antibodies and an irrelevant rabbit anti-mouse IgG (Pierce), respectively. Western immunoblot analyses were then performed with syntaxin 10 antibodies.
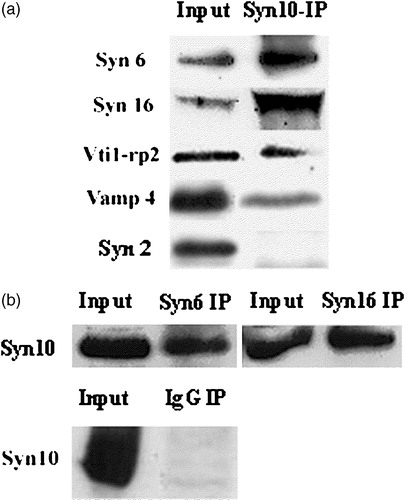
Figure 3. Syntaxin 10 antibody does not inhibit Shiga toxin B-(sulf)2 sulfation. Syntaxin 10 or syntaxin 16 antibodies, either heat-inactivated at 100°C for 2 min (boiled) or otherwise (normal), were added to standard transport assay set ups as described in Materials and Methods. Control indicates a standard reaction without any antibodies. 35S-labeled Shiga B were resolved with a 15% modified Laemmli peptide separation PAGE gel, and blotted onto nitrocellulose membranes. The membrane is then subjected to phosphoimaging analysis. A representative autoradiogram is shown. The graph represents quantitative analysis of several independent experiments. Band intensities were quantified by densitometry, and expressed as percentage of the respective controls (which were set arbitrarily as 100%). P<0.01 in the case of syntaxin 16 antibody added, compared to control.
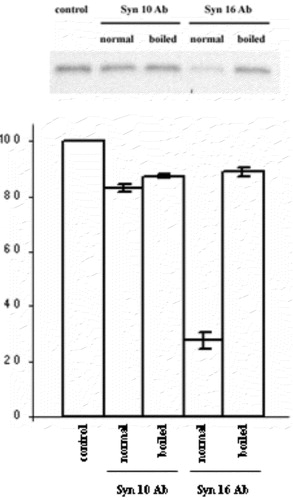
Figure 4. Dominant-negative form of syntaxin 10 does not inhibit Shiga B transport to the TGN. Cells transiently transfected with constructs expressing the cytoplasmic domain of syntaxin 10 (Syn10cyto) or syntaxin 16 (Syn16cyto) were fixed with 4% paraformaldehyde and incubated with antibody against syntaxins 10 or 16 (rabbit) and mouse monoclonal antibody against Shiga B, followed by Texas-red or FITC-labeled secondary antibodies. Bar = 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
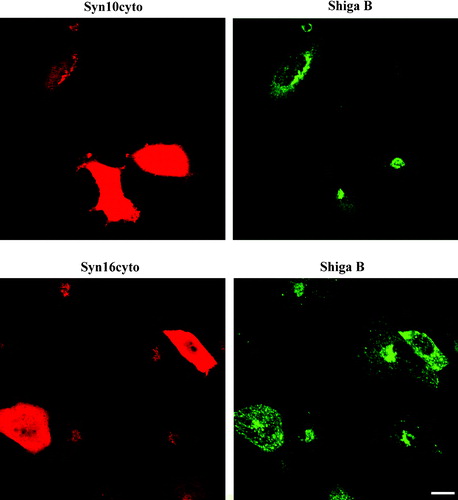
Figure 5. Effects of knockdown of syntaxin expressions on TGN morphology and on the localization of other TGN syntaxins: (a). Cell were transiently transfected with either siRNA oligomers for syntaxin 10, syntaxin 16 or glutathione S-transferase (as a control) for 48 h and the protein levels in the cell lysates were analyzed by immunoblotting for syntaxin 10 and 16, respectively. Immunoblotting for tubulin indicates roughly equivalent protein loading in each lane; (b). Cells were transiently transfected with the respective siRNA oligomers for 48 h. Cell were then fixed with 4% paraformaldehyde and incubated with primary antibody against syntaxins 10 or 16 (rabbit), with mouse monoclonal antibody against syntaxin 6, or mouse anti-syntaxin 16 antiserum, followed by Texas-red or FITC-labeled secondary antibodies. Arrows indicate cells where syntaxin 16 knockdown is incomplete or minimal. Note that the syntaxin 6 staining pattern in these cells remained perinuclear. Comparatively, the perinuclear staining of syntaxin 6 in cells with significant syntaxin 16 knockdown became diffused (arrowheads). However, the perinuclear staining of syntaxin 10 is largely unaffected in syntaxin 16 knockdown cells. Bar = 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
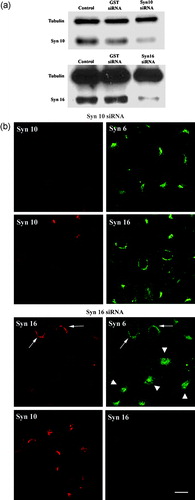
Figure 6. Knockdown of syntaxin 10 expressions has no effect on Shiga B transport to the TGN. Cells were transiently transfected with syntaxin 10 (Syn10) or syntaxin 16 (Syn 16) siRNA oligomers for 48 h. Cells were then incubated with Shiga B fragment (5 µg/ml) for 1 h and then fixed with 4% paraformaldehyde. They were incubated with primary antibody against syntaxins 10 or 16 (rabbit) and mouse monoclonal antibody against Shiga B, followed by Texas-red or FITC-labeled secondary antibodies. In cells with efficient syntaxin 16 knockdown, Shiga B staining remained spotty and early-endosome-like. Arrows indicated cells where syntaxin 16 knockdown was not particularly effective, and syntaxin 16 staining is still visible in these cells. Note that Shiga B transport to the perinuclear TGN patch was not impaired in these cells. Bar = 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
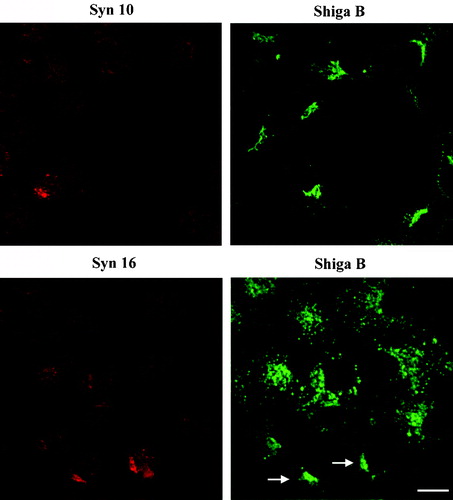
Figure 7. Knock down of syntaxin 10 or syntaxin 16 expressions has no effect on VSVG transport to the cell surface and little effect on the localization of the mannose 6-phosphate receptor: (a) Left panels: Cells were transiently transfected with syntaxin 10 (Syn10) or syntaxin 16 (Syn 16) siRNA oligomers for 48 h and then infected with VSVts045 for 6 h as described in Materials and Methods. Cells were then fixed with 4% paraformaldehyde and incubated with primary antibody against syntaxins 10 or 16 (rabbit) and mouse monoclonal antibody against VSVG, followed by Texas-red or FITC-labeled secondary antibodies. Note that although not all cells are infected with the virus and therefore expressing VSVG, neither normal nor syntaxin 10/16 knockdown cells are impaired in terms of surface VSVG expression. Bar = 10 µm. (b). Right panels: Cells were transfected with syntaxin 10 (Syn10) or syntaxin 16 (Syn 16) siRNA oligomers for 48 h and double-labeled with antibody against syntaxins 10 or 16 (rabbit) and mouse monoclonal antibody against M6PR, followed by Texas-red or FITC-labeled secondary antibodies. Bar = 10 µm; (b). Changes in the morphology of M6PR with varying degree of syntaxin 10 knockdown. M6PR staining (green) is more diffuse in cells with higher degree of syntaxin 10 (red) knock down, but remains distinctly perinuclear. Figures 7A and 7B are reproduced in colour in Molecular Membrane Biology online.
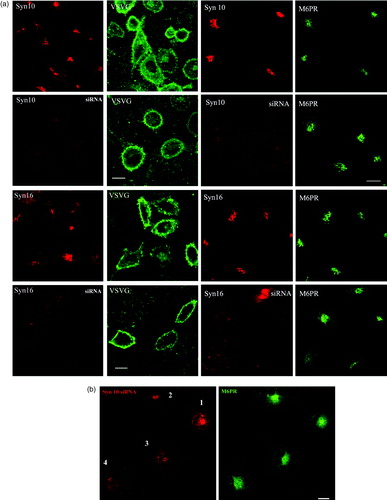
Figure 8. Syntaxin 10 interacts with syntaxin 12/13 and its depletion alters the steady state localization of TfR: (a). HeLa cell lysates were subjected to immunoprecipitation with antibodies against syntaxin 12/13, 10 or 16. Immunoprecipitates were eluted, resolved on 8% SDS-PAGE gels, and subjected to Western immunoblot analyses with antibodies against Vps45. Input is 1/7.5 of the lysate used for the immunoprecipitation. Representative blots are shown; (b). Cells were transiently transfected with the respective siRNA oligomers for 48 h. Cell were then incubated with culture supernatants (diluted 1:10 with basal RPMI medium) of the TfR monoclonal antibody producing Okt9 hybridoma for 20 min on ice. For the assessment of cell surface TfR levels (surface), cells were then washed extensively, fixed with 4% paraformaldehyde and incubated with primary antibodies against syntaxins 10 or 16, followed by Texas-red or FITC-labeled secondary antibodies. For the assessment of internal TfR levels (internal), cells were washed extensively, immersed in complete RPMI medium, transferred to 37°C, and incubated for 30 min. Cell were then washed, fixed with 4% paraformaldehyde and incubated with primary antibodies against syntaxin 10, followed by Texas-red or FITC-labeled secondary antibodies. Arrowheads indicate cells where syntaxin 10 or 16 knockdown is incomplete or minimal. Bar = 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
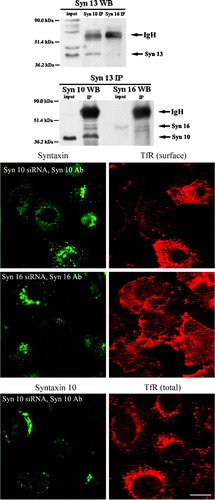
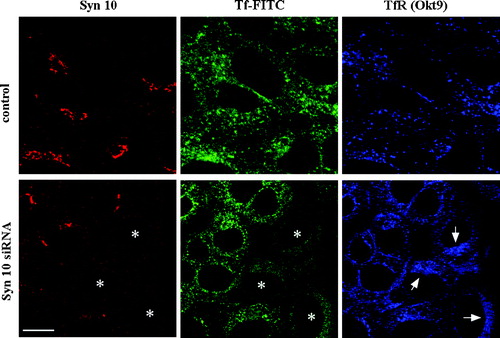
Figure 9. Syntaxin 10 knock down increases intracellular accumulation TfR at a perinuclear region. Cells were transiently transfected with syntaxin 10 (Syn10) siRNA oligomers for 48 h. Transfected cells were allowed to internalized transferrin-FITC for 1 h at 18°C, followed by another 30 min of incubation in plain medium at 18°C. Cells were then shifted to 37°C for varying times before fixing and labeled with mouse monoclonal antibody against transferrin (Okt9) and the rabbit polyclonal antibody against syntaxin 10. This is followed by incubations with Cy5 conjugated goat anti-mouse IgG and Texas-red conjugated goat anti-rabbit IgG. Shown are cells after 5 min incubation at 37°C, where the transferrin-FITC staining is still obvious. Asterisks (*) indicate 3 cells with clear syntaxin 10 knockdown and arrows point to the perinuclear accumulation of TfR (labeled by Okt9) in these cells. Bar = 10 µm.