Figures & data
Figure 1. Schematic representation of K+ channel topologies of the constructs made and sequence comparison of the C-terminus. (A) Bar representations of hKv1.4, hKv1.1, hKv1.2, 4/1C chimæra with the N terminus and TM body of hKv1.4 and the C-terminus from hKv1.1, the 4/2C chimæra with the N-terminus and TM body from hKv1.4 and the C-terminus from hKv1.2, the C-terminal truncation constructs, 1.4ΔC40 and 1.4ΔC23 representing a deletion of 40 and 23 amino acids from the C-terminus of hKv1.4 respectively. Filled circles at the proximal ends of the bar representations symbolize the N-terminal ball present in fast inactivating channels like hKv1.4 and its derivative chimæras. Crosses at the N-terminal region of the bar denote the tetramerization (T1) domain. (B) Sequence comparison of the amino acid residues starting from the S5 TM domain to the C-terminus in hKv1.1, hKv1.2 and hKv1.4. The vertical arrow depicts the splice point of the chimæric constructs. Shading represents the conservation of amino acid residues among the three potassium channels discussed here.
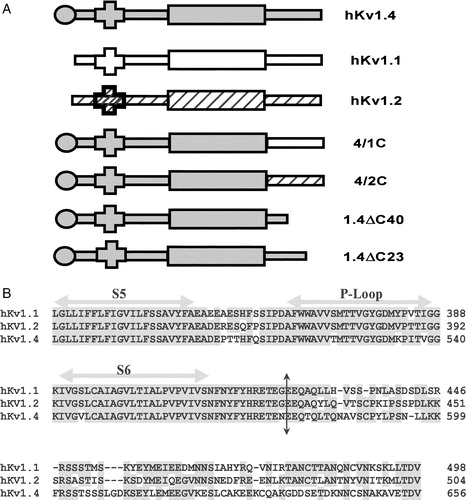
Figure 2. Potassium currents through channels expressed in Xenopus laevis oocytes on membrane depolarization. Currents elicited in oocytes expressing (A) non-inactivating hKv1.1; (B) fast inactivating hKv1.4; (C) 4/1C chimæra; (D) slower and incompletely inactivating 4/2C chimæra, upon depolarization from a holding potential of −80 mV and subject to a 1 s hyperpolarizing prepulse of −120 mV prior to depolarizing to potentials indicated against the traces; (E) currents elicited upon depolarization of 4/2C from −80 mV in steps of +10 mV for 3.6 s. The inter-episode time used in this protocol was 10 s. The voltage pulse protocol has been shown in panel F.
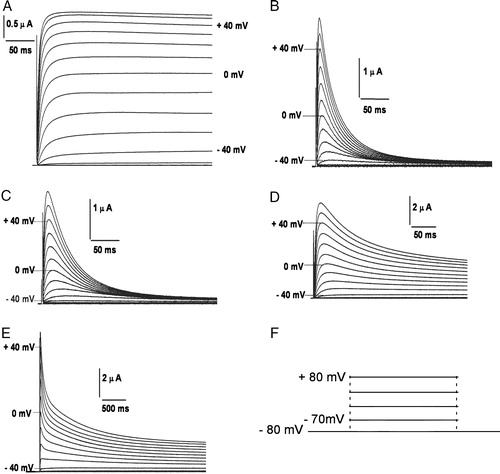
Figure 3. Kinetic analysis of currents. (A) Voltage-dependence of K+ currents: voltage-dependent activation of hKv1.4 (○), 4/1C (•) and 4/2C (▵). The curves through the data were best fit to Boltzmann functions as described in experimental procedures; (B) activation kinetics: time constants for the activation of the currents for hKv1.4 (○), 4/1C (•) and 4/2C (▵), obtained by fitting the currents to a double exponential function and plotting the faster time constant as a function of trans-membrane potential; (C) inactivation kinetics: inactivation time constants for hKv1.4 (○), 4/1C (•) and 4/2C (▵), obtained by fitting the currents to a double exponential function and plotting the slower time constant against the membrane potential. The error bars for all data sets represent SEM. (n≥8).
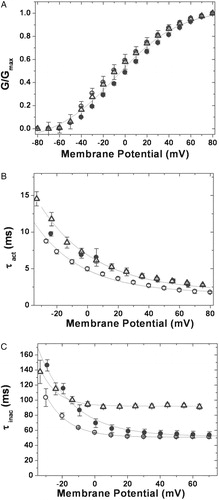
Figure 4. Voltage dependence of inactivation. Steady state inactivation kinetics: steady state inactivated channels obtained by delivering a series of depolarizing prepulses ranging from −80 mV to +50 mV until a steady state of inactivation is achieved, were stepped to a test potential of +40 mV (C). The currents elicited upon depolarization are shown for hKv1.4 (A) and 4/2C chimæra (B). The normalized peak currents at the test potential of +40 mV are plotted as a function of the prepulse potentials and fitted to Boltzmann functions (D). The V1/2 for 4/2C was estimated by renormalizing to the minimum value, thereby analyzing only the inactivating portion of the currents (represented as the dotted line). The calculated slope factors were 5.32±0.24 for hKv1.4 and 4/1C and 5.96±0.62 for the 4/2C construct. The renormalized curve for 4/2C had a slope factor of 6.74±0.32. The error bars for all data sets represent SEM. (n≥8).
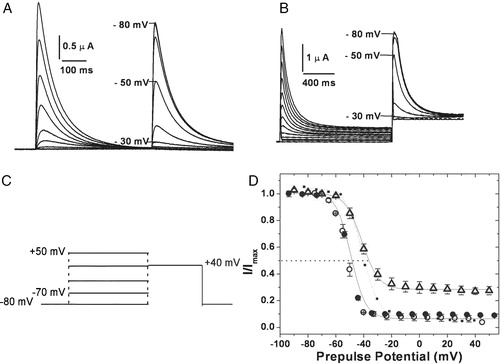
Figure 5. External potassium and TEA independence of inactivation kinetics. (A) Fold change in τinac upon varying the external K+ concentrations: the time constant of inactivation at 0 mV estimated from oocytes expressing 4/2C channels in the presence of 10, 48, 73 and 96 mM [K+]out was normalized using the τinac value at 2 mM [K+]out. These values were then plotted against the [K+]out values and fit to a straight line function. (B) Fold change in τinac in the presence of different concentrations of externally applied TEA: the time constant of inactivation was calculated for currents recorded from oocytes expressing 4/2C channels with 0, 10, 20 and 40 mM TEA in the bath solution at 0 mV. The τinac values estimated for these currents recorded in the presence of 10, 20 and 40 mM TEA were normalized using the τinac value estimated in the control oocyte with no TEA in the bath. These values were then plotted against the concentration of TEA and fit to a straight line function.
![Figure 5. External potassium and TEA independence of inactivation kinetics. (A) Fold change in τinac upon varying the external K+ concentrations: the time constant of inactivation at 0 mV estimated from oocytes expressing 4/2C channels in the presence of 10, 48, 73 and 96 mM [K+]out was normalized using the τinac value at 2 mM [K+]out. These values were then plotted against the [K+]out values and fit to a straight line function. (B) Fold change in τinac in the presence of different concentrations of externally applied TEA: the time constant of inactivation was calculated for currents recorded from oocytes expressing 4/2C channels with 0, 10, 20 and 40 mM TEA in the bath solution at 0 mV. The τinac values estimated for these currents recorded in the presence of 10, 20 and 40 mM TEA were normalized using the τinac value estimated in the control oocyte with no TEA in the bath. These values were then plotted against the concentration of TEA and fit to a straight line function.](/cms/asset/5812f069-8ddd-423f-859e-97cb3d18660d/imbc_a_119049_f0005_b.jpg)
Figure 6. Recovery from inactivation. (A) Pulse protocol used: the membrane potential was stepped from a holding potential of −80 mV to −10 mV for 750 ms. The cell was then hyperpolarized to −100 mV for varying periods of time before stepping back to the test potential of −10 mV. (B) The currents obtained in oocytes expressing 4/2C when subjected to the pulse protocol described above. (C) The fraction of channels recovered was plotted as a function of recovery time and fitted to a single exponential function. The 4/2C (▵) chimæra data fit with a single time constant of 1678 ms as against 1953 ms for channels encoded by hKv1.4 (○), and 4/1C (•) channels. Only one in every three data points recorded are represented in order to retain clarity of the figures. The error bars for all data sets represent SEM. (n≥8).
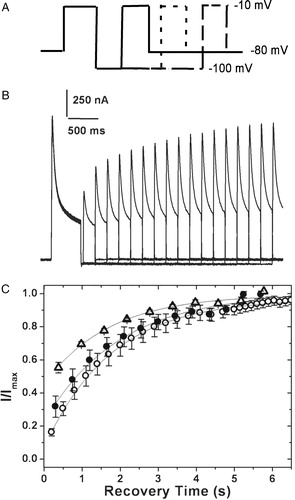
Figure 7. C-terminal truncation constructs. (A) Secondary structure prediction: a secondary structure prediction of the C-terminal residues of hKv1.1, hKv1.2 and hKv1.4, done as described in experimental procedures. Shaded regions represent predicted helical stretches. Arrows indicate residues where stop codons were introduced while generating the 1.4ΔC23 and 1.4ΔC40 constructs. The absence of the predicted helical structure in hKv1.2 is to be noted. Currents elicited in oocytes expressing the 1.4ΔC40 construct upon depolarization to (B) 320 ms and (C) 7 seconds. (D) 1.4ΔC23 construct in response to depolarizing pulses from a holding potential of −80 mV to the potentials indicated against the traces, with an interval of 10 s between two successive sweeps.
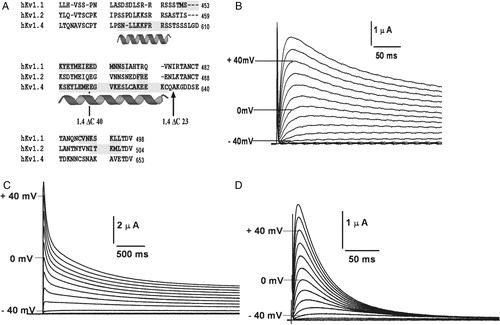
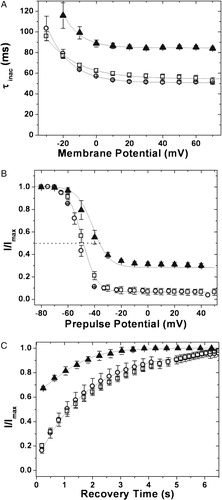
Figure 8. Inactivation kinetics and recovery from inactivation of the C-terminal truncation constructs. (A) Inactivation kinetics: inactivation time constants for hKv1.4 (○), 1.4ΔC40 (▴) and 1.4ΔC 23 (□), obtained by fitting the currents to a double exponential function and plotting the slower time constant against the membrane potential. The error bars for all data sets represent SEM. (n≥8). (B) Voltage dependence of inactivation: steady state inactivation kinetics: steady state inactivated channels obtained by delivering a series of depolarizing prepulses ranging from −80 mV to +50 mV until a steady state of inactivation is achieved, were stepped to a test potential of +40 mV. The normalized peak currents at this potential are plotted as a function of the prepulse potentials and fitted to Boltzmann functions. The mid points of inactivation obtained thus were −43.01 mV and −52.0±0.1 mV for 1.4ΔC40 and hKv1.4 respectively. The V1/2 for 1.4ΔC40 was estimated by renormalizing to the minimum value, thereby analyzing only the inactivating portion of the currents. The calculated slope factors were 5.32±0.24 for hKv1.4, and 1.4ΔC23 and 5.96±0.62 for the 1.4ΔC40 construct. The renormalized curve for 1.4ΔC40 had a slope factor of 6.74±0.32. The error bars for all data sets represent SEM. (n≥8). (C) Recovery from inactivation: the membrane potential was stepped from a holding potential of −80 mV to −10 mV for 750 ms. The cell was then hyperpolarized to −100 mV for varying periods of time before stepping back to −10 mV. The fraction of channels recovered was plotted as a function of recovery time and fitted to a single exponential function. The 1.4ΔC40 (▴) construct data fit with a single time constant of 1096 ms as against 1953 ms for channels encoded by hKv1.4 (○) and 1.4ΔC 23 (□) constructs. Only one in every three data points recorded are represented in order to retain clarity of the figures. The error bars for all data sets represent SEM. (n≥8).