Figures & data
Figure 1. Excitation- and emission-spectra of CFP-Munc 18-1, Munc 18-1-CFP and YFP-syntaxin. CHO-cells were transfected with CFP-Munc 18-1 (——), Munc 18-1-CFP (-------) or YFP-syntaxin (—•—•—). Thirty-six hours later cells were lysed and excitation (A) and emission (B) spectra were measured using an Aminco Bowman Series II fluorescence spectrophotometer (Polytec, Waldbronn, Germany). Intensity in arbitrary units (a.u.) is plotted against the wavelength (nm).
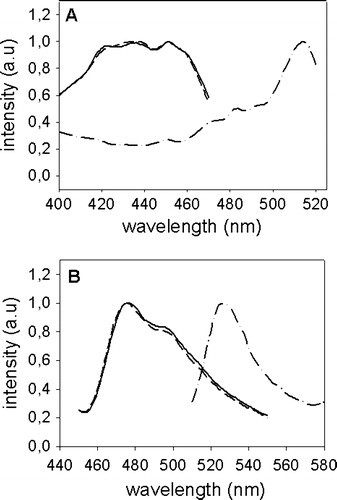
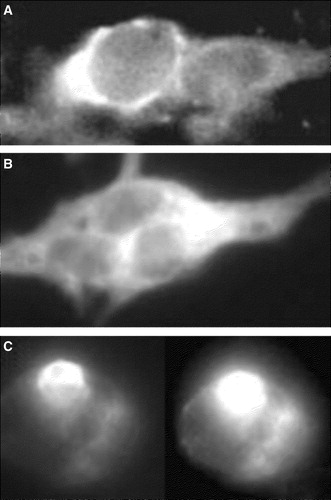
Figure 2. Localization of Munc 18-1 and syntaxin in CHO-cells. CHO-cells were transfected with CFP-Munc 18-1 (A), YFP-syntaxin wild-type (B) or YFP syntaxin mutant L165A, E166A (C). Thirty-six hours later intracellular localization of proteins was visualized with confocal microscopy.
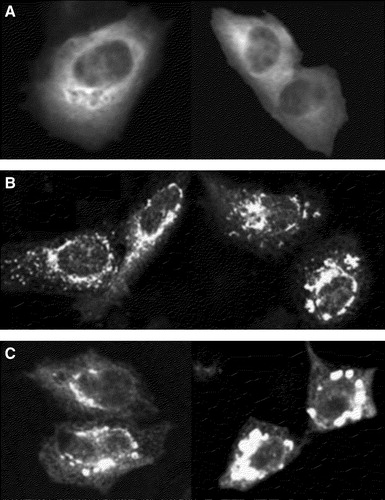
Figure 3. Colocalization of YFP-syntaxin with Golgi-markers in CHO-cells. CHO-cells were transfected with YFP-syntaxin wild-type. Thirty-six hours later cells were costained with TRITC-WGA (A–C) or Bodipy-ceramide (D–E) and intracellular localization of proteins and marker-stains was visualized with confocal microscopy. A and D: marker stains TRITC-WGA (A) and Bodipy-ceramide (D). B and E: YFP-syntaxin. C and F = pixels which are labelled both with YFP-syntaxin and the marker-stain.
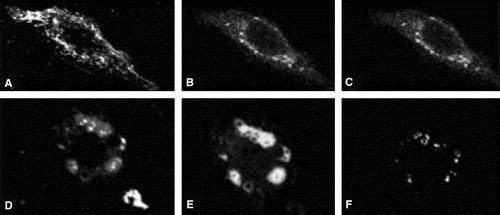
Figure 4. Localization of Munc 18-1 and syntaxin in double transfected CHO-cells. (A–C) Munc 18-1 does colocalize with syntaxin wild-type. CHO-cells were double transfected with CFP-Munc 18-1 and YFP-syntaxin wild-type. Thirty-six hours later intracellular localization of proteins was visualized with confocal microscopy. (A) YFP channel, (B) CFP channel, (C) merged images. The insets are from a different optical field. (D–F) Munc 18-1 does not colocalize with the syntaxin mutant L165A, E166A. CHO-cells were double transfected with CFP-Munc 18-1 and YFP-syntaxin mutant L165A, E166A. Thirty-six hours later intracellular localization of proteins was visualized with confocal microscopy. (A) YFP channel, (B) CFP channel, (C) merged images.
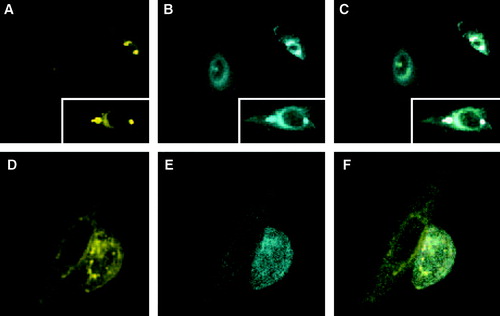
Figure 5. FRET with CFP-Munc 18-1 and YFP-syntaxin wild-type in CHO-cells. CHO-cells were double transfected with CFP-Munc 18-1 and YFP-syntaxin wild-type. Thirty-six hours later localization of proteins was visualized with a CCD camera. (A) CFP-channel, (B) YFP-channel, (C) FRET-channel. To correct for the bleed-through Net-FRET was calculated as described in ‘Methods’. Images were then pseudo-colored. The color bar represents relative degree of Net-FRET within the cells.
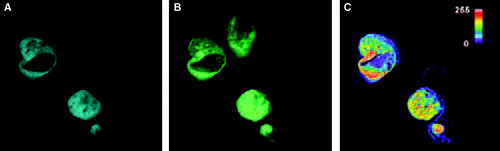
Figure 6. Localization of CFP-Munc 18-1 and YFP-syntaxin in MDCK-cells and FRET-analysis with acceptor photobleaching. MDCK-cells were double transfected with CFP-Munc 18-1 and YFP-syntaxin wild-type. Thirty-six hours later localization of proteins was visualized with a CCD camera as decribed in . The acceptor fluorophor was then photobleached with YFP-excitation for 3 min and additional images were taken at all wavelengths. Upper panel: before bleaching. Lower panel: after bleaching. (A, D) CFP-channel, (B, E) YFP-channel, (C, F) FRET-channel corrected for bleed-through (Net-FRET calculated as described in ‘Methods’).
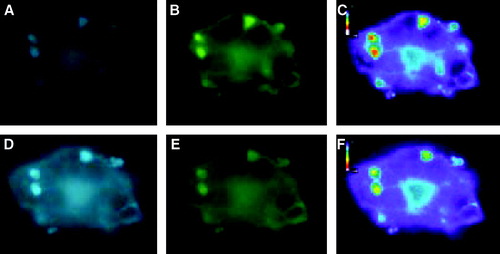
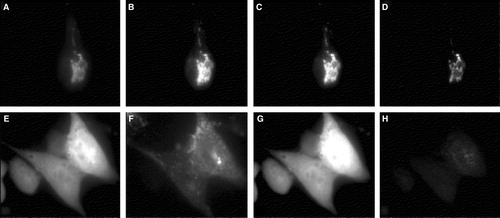
Figure 7. Comparison of FRET with syntaxin mutant L165A, E166A. CHO-cells permanently expressing Munc 18-1-CFP were transfected with either wild-type YFP-syntaxin (upper panel) or the YFP-syntaxin mutant L165A, E166A (lower panel). (A, E) CFP-channel, (B, F) YFP-channel (C, G) Raw-Fret as observed in the FRET-channel, (D, H) Net-FRET signals were calculated as described in ‘Methods’. More than 50 images were analyzed in this way and in each case Munc 18-1-CFP showed FRET with syntaxin wild-type, but not with syntaxin L165A, E166A.