Figures & data
Figure 2. Surface pressure increase after the injection of AmB beneath the air-water interface. Each arrow indicates the injection of 10 µl of AmB solution into 12 ml of buffer. After single injection the AmB concentration in the subphase was increased by 0.9 µM. The solution of AmB was prepared either in DMSO (1) or in water alkalized with KOH to pH 12 (3). Trace No. 2 represents the time-dependency of the surface pressure after injection of the same volume of pure DMSO. Inset shows comparison of the effects of AmB injected in the form of solution in DMSO and water pH 12 on surface pressure changes.
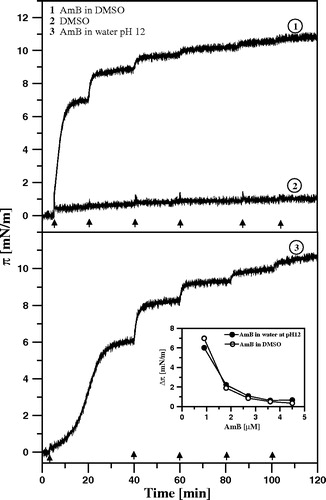
Figure 3. Isotherms of compression of monomolecular layers formed with pure AmB (solid line) and pure DPPC (dotted line).
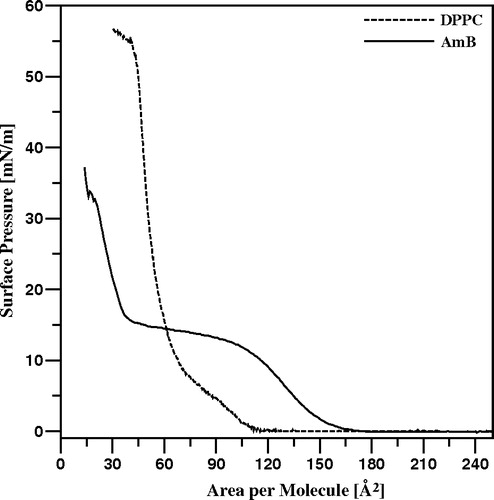
Figure 4. Surface pressure increase after the injections (marked with arrows) of 10 µl of AmB solution in DMSO (panel A) or in water pH 12 (panel B) into the air-water subphase (12 ml), beneath the monomolecular layers at the surface pressure 22 mN/m, formed with: DPPC (1), DPPC + Cholesterol 1:1 (2) and DPPC + Ergosterol 1:1 (3). Each injection of AmB increased its concentration by 0.9 µM. Panel C presents the effect of the single injection. Temperature 26°C.
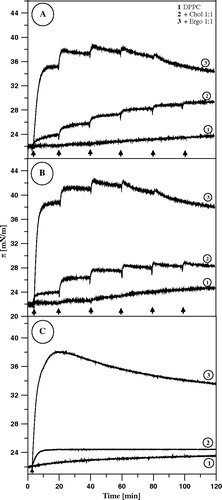
Figure 5. Surface pressure increase after the injection (marked with an arrow) of 10 µl of AmB solution in DMSO into the air-water subphase (12 ml), beneath the monomolecular layers at the surface pressure 22 mN/m, formed with: DPhPC, DPPC and EYPE (indicated). The final concentration of AmB was 0.9 µM. Temperature 26°C.
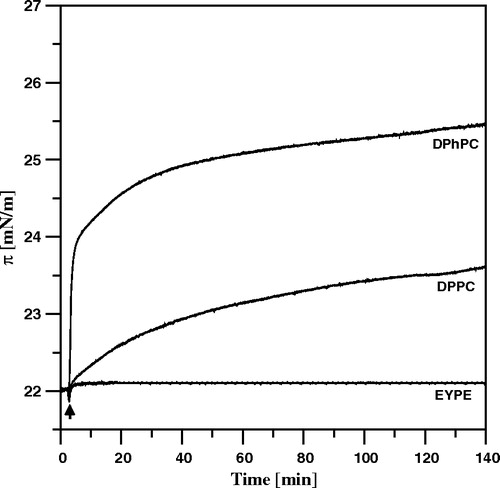
Figure 6. Polarized ATR-FTIR absorption spectra recorded from Langmuir-Blodgett monomolecular films of AmB deposited at two sides of a Ge crystal. The films were deposited from the monomolecular layer of AmB at the air-water interface at the surface pressure 10 mN/m. The spectrum recorded with parallel polarisation of the electric vector of radiation with respect to the plane of incidence is plotted with the solid line and perpendicular polarisation with the dotted line. The inset drawing presents orientation of the AmB molecule deposited at the plate of crystal, that corresponds to the tilt angle of the molecular axis with respect to the axis normal to the plane of the crystal (z axis) θ = 90°. The assignment of the main spectral bands and the integration limits of the spectral band corresponding to the C = C stretching vibrations are shown.
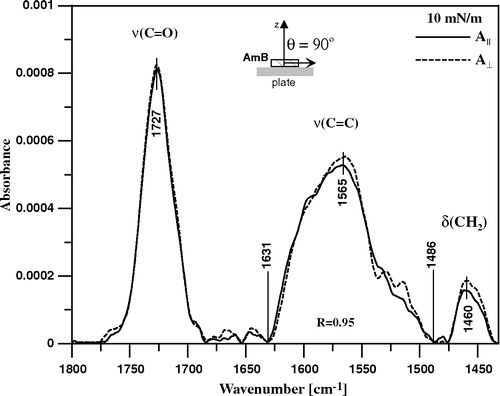
Figure 7. ATR-FTIR absorption spectra recorded from the Langmuir-Blodgett monomolecular films deposited at two sides of a Ge crystal from lipid monomolecular layer of DPPC formed at the air-water interface, compressed and stabilized at the surface pressure 22 mN/m (dashed line) and the DPPC film deposited to the crystal after the injection of 10 µl of water solution of AmB (pH 12) into 12 ml of the subphase (solid line). Final concentration of AmB in the subphase 0.9 µM. The central part of the spectra is also presented on the 10-fold expanded absorption scale.
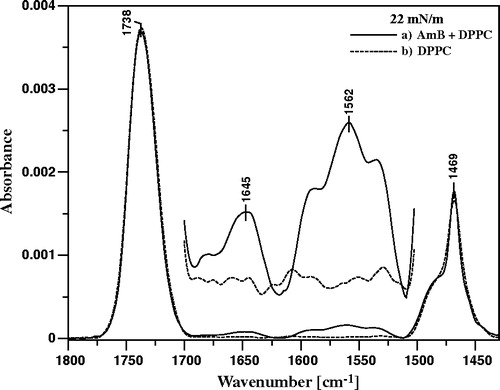
Figure 8. ATR-FTIR absorption spectra recorded from the AmB-containing Langmuir-Blodgett monomolecular films deposited at two sides of a Ge crystal from the lipid monomolecular layers at the air-water interface compressed to the surface pressure 22 mN/m composed of: (A) pure DPPC, (B) DPPC + Cholesterol 1:1 (by mole) and (C) DPPC + Ergosterol 1:1 (by mole). The electric vector of the IR radiation was polarized parallel (dashed line) and perpendicular (solid line) with respect to the plain of incidence. The Langmuir-Blodgett monomolecular films deposited from the lipid monolayers 20 min after the injection of 10 µl of the water solution of AmB (pH 12) into 12 ml of the subphase. Final concentration of AmB in the subphase was 0.9 µM.
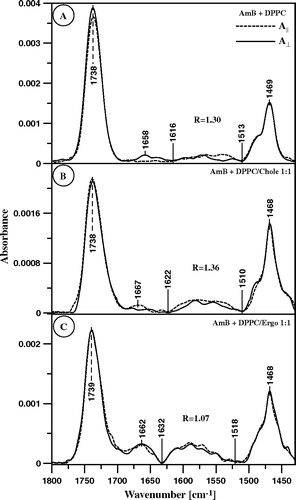
Table I. Orientation angle (θ) of the long molecular axis of AmB bound to monomolecular layers formed with DPPC, DPPC and cholesterol or DPPC and ergosterol, with respect to the axis normal to the plane of the film, determined on the basis of linear dichroism measurements (see ) and Equation 4.
Figure 9. ATR-FTIR absorption spectra recorded from the Langmuir-Blodgett monomolecular films deposited at two sides of a Ge crystal from the lipid monomolecular layer of DPPC formed at the air-water interface, compressed to the surface pressure 22 mN/m (dashed line) and the DPPC film deposited to the crystal after the injection of 10 µl of water solution of AmB (pH 12) into 12 ml of the subphase (solid line). Final concentration of AmB in the subphase 0.9 µM. The spectral region presented corresponds to the stretching vibrations of CH3 groups (νs 2881 cm−1, νas 2959 cm−1) and CH2 groups (νs 2850 cm−1, νas 2919 cm−1).
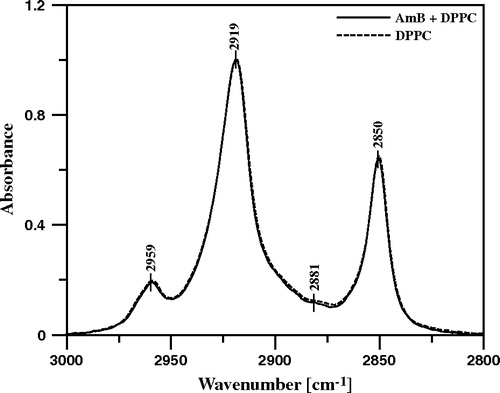
Table II. Mean orientation angle (θ) of the molecular axis along the CH2 alkyl chains of DPPC in monomolecular layers formed with DPPC, DPPC and cholesterol or DPPC and ergosterol, with respect to the axis normal to the plane of the film, determined on the basis of linear dichroism measurements (Equation 3). The Table also presents the orientation angle in the case of the lipid films deposited from monolayers after injection of 0.9 M AmB into the subphase and deposited after time required for equilibration of the system. The angle α has been taken arbitrary as 90o according to the literature data Citation[25].